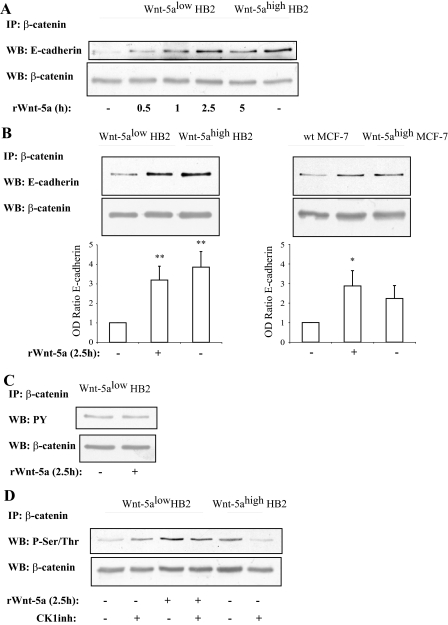
FIGURE 3.
A, time kinetics of Wnt-5a inducedβ-catenin/E-cadherin complex formation indicates that it is a rapid and specific process unrelated to general protein expression levels (n = 3). B, β-catenin/E-cadherin complex formation is potentiated by Wnt-5a signaling as judged by co-immunoprecipitations of β-catenin/E-cadherin complexes from human mammary epithelial cell line HB2 (left) and human breast cancer cell line MCF-7 (right) cells with varying Wnt-5a expression levels or in cells treated with recombinant Wnt-5a. The blot shown is representative of several separate experiments and OD measurements of the band intensities were performed to quantify the differences (lower panel; histograms). Error bars indicate S.E., n = 8, n = 5; *, p < 0.05; **, p < 0.01. C, tyrosine phosphorylation of β-catenin is not affected by Wnt-5a stimulation, whereas serine/threonine phosphorylation is (D) as judged by immunoprecipitation of β-catenin and Western blot (WB) using a total Tyr(P) or Ser/Thr antibodies. Inhibition of the serine/threonine kinase CKI, with the casein kinase 1 α/ε inhibitor IC261, disrupts the Wnt-5a-induced Ser/Thr phosphorylation of β-catenin, whereas cells lacking Wnt-5a remain Ser/Thr phosphorylated on β-catenin (D). The CKI inhibitor was added for 1 h at the end of the incubation with rWnt-5a (n = 5). Wt, wild type.