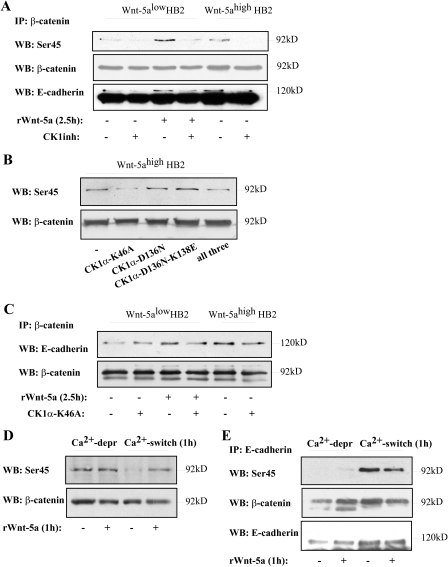
FIGURE 5.
A, Wnt-5a/CKI specifically induces Ser-45 phosphorylation of β-catenin. This correlates with increased levels of co-immunoprecipitated E-cadherin (n = 3). The lower band in the E-cadherin reblot is β-catenin. B, transfection of Wnt-5ahigh HB2 cells with three different variants of dominant negative kinase-deficient mutants of CKIα (26): K46A, D136N, and D136N-K138E or all three. Overexpression of CKIα-K46A disrupts the Wnt-5a-induced Ser-45 phosphorylation of β-catenin. C, overexpression of CKIα-K46A also disrupts the Wnt-5a-induced increase in β-catenin/E-cadherin complex formation. D, initiation of cell-cell adhesion per se does not induce Ser-45 phosphorylation of β-catenin. Ca2+-dependent cell-cell adhesion was initiated by performing a Ca2+-switch experiment (see “Experimental Procedures”). Wnt-5alow HB2 cells were allowed to re-adhere for 1 h after the Ca2+ switch in the absence or presence of rWnt-5a. Cells where cell-cell contacts had recently been disrupted and cells treated with rWnt-5a show β-catenin Ser-45 phosphorylation (also see C). In sharp contrast, initiation of de novo cell-cell contacts per se does not lead to Ser-45 phosphorylation, whereas addition of rWnt-5a does (n = 3). E, the pool of Ser-45-phosphorylated β-catenin seen after cell-cell contact disruption in B is not associated to E-cadherin complexes as shown by co-immunoprecipitations of E-cadherin after Ca2+ deprivation. In sharp contrast, the E-cadherin-associated β-catenin after a Ca2+ switch (re-adherence) is Ser-45 phosphorylated. As described in the text it is not possible to compare the amount of coprecipitated β-catenin using the (SHE 78-7) E-cadherin ectodomain antibody and this might explain why we do not see an increased Ser-45 phosphorylation after Wnt-5a stimulation compared with unstimulated cells in this particular set of experiments. The lower band in the E-cadherin reblot is β-catenin. WB, Western blot; IP, immunoprecipitate.