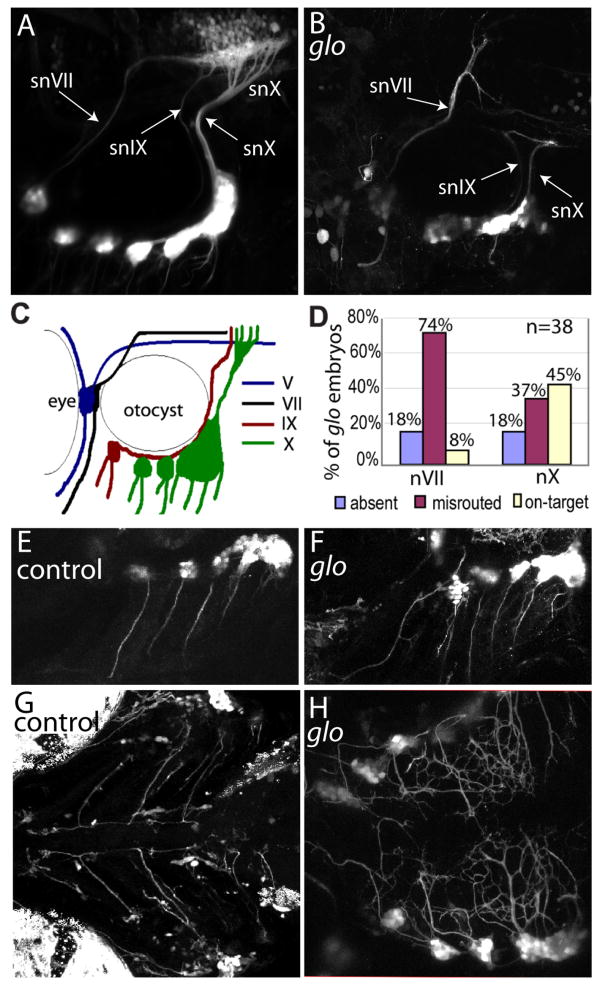
Figure 1. Absence of cdh2 function in glo mutant embryos disrupts axon pathfinding of the epibranchial ganglia.
Confocal Z-stacks comparing control CSG (A,E,G) and glo CSG (B,F,H) at 4dpf. In all panels, rostral is to the left. A, B, Lateral views of 4dpf control (A) and homozygous glom117;tg(p2xr3.2:eGFPsl1) (B) embryos showing defects in the gVII, gIX and gX central axonal projections (labeled snVII, snIX and snX, respectively). C, Schematic illustration of GFP-labeled nerves in A. D, Graph representing the percentage of glo mutant embryos displaying normally projecting, aberrantly projecting or absent gVII or gX axons. 38 homozygous glo m117;tg(p2xr3.2:eGFPsl1) embryos from two clutches were examined under confocal microscopy and their central projections were scored. E and F, Lateral views of tg(p2xr3.2:eGFP) control (E) and glo (F) embryos showing bundles of axons extending ventrally from each epibranchial ganglia in both control and glo mutant embryos. G and H, Ventral views of control (G) and glo; tg(p2xr3.2:eGFPsl1) mutant (H) embryos showing the bilateral ganglia extending peripheral processes to the ventral midline. In glo embryos the processes defasciulate as they reach the midline and fail to turn and extend along the rostral-caudal axis. snVII, snIX and snX are abbreviations for gVII, gIX and gX nerves, respectively.