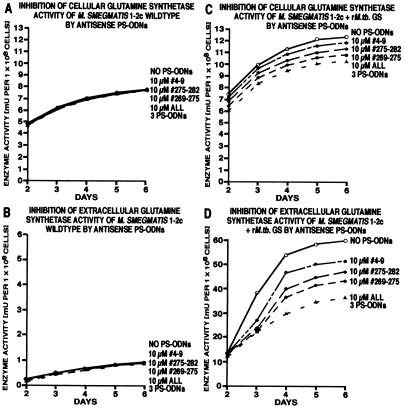
Figure 2.
Inhibition of glutamine synthetase activity in M. smegmatis 1–2c wild type and its recombinant isotype expressing the M. tuberculosis glutamine synthetase. Duplicate M. smegmatis cultures were grown for 2 days in 1–2 ml of 7H9 medium alone and thereafter in the absence or presence of antisense PS-ODNs, individually or combined, at concentrations of 10 μM. At each time point indicated, cultures were harvested and analyzed for glutamine synthetase activity by the transfer assay as described (2). Note that the cellular enzyme activity of the recombinant strain is a mixture of two enzyme activities—the endogenous glutamine synthetase (60%) and recombinant M. tuberculosis glutamine synthetase (40%). In contrast, the extracellular enzyme activity of the recombinant strain is almost exclusively M. tuberculosis glutamine synthetase (≥99%). All data points had a SD of ≤15%.