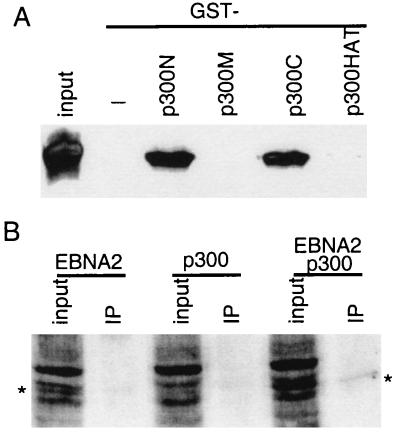
Figure 4.
EBNA2 interacts with p300 in vitro and in vivo. (A) EBNA2 interacts with amino- and carboxyl-terminal of p300 in vitro. GST fusions to the p300 amino-terminal (amino acids 1–585, p300N), middle (amino acids 766–1571, p300M), carboxyl-terminal (amino acids 1572–2370, p300C), or HAT (amino acids 1195–1701, p300HAT) domains were expressed in E. coli, purified by using glutathione-Sepharose beads, and incubated with His-tagged full-length wild-type EBNA2 protein that has also been purified from E. coli. EBNA2 binding to GST-p300 beads was assayed by Western blot with an EBNA2-specific mAb (PE2) after SDS/PAGE of proteins that bound to the beads. The amount of protein used in the binding assays was five times that shown as input. (B) EBNA2 coimmune-precipitates with p300 from 293 T cells overexpressing both proteins. EBNA2, flag-tagged p300, or both were transfected into 293 T cells. Cell extracts were incubated with M2 beads to immune-precipitate flag-tagged p300, and EBNA2 was detected by Western blot with PE2 mAb. P300 was precipitated from 10 times the amount of lysate shown as input.