Figure 6.
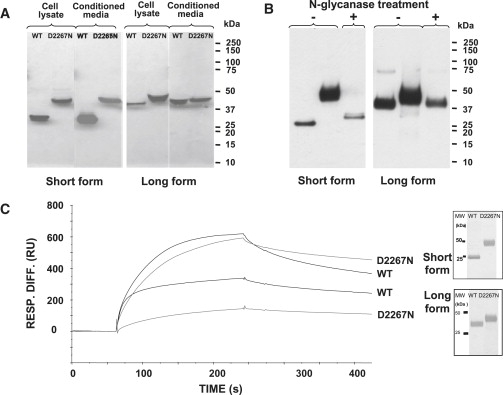
Biochemical Analyses of the Wild-Type and Mutant Aggrecan G3 Proteins
(A) The effect of the D2267N mutation on the secretion of the aggrecan G3 domain. SDS-PAGE and western-blot analysis of conditioned media and cell lysates from 293-EBNA cells transiently transfected with the aggrecan G3 domain constructs (WT and mutant). Recombinant proteins were resolved on 4%–12% Bis-Tris gel under reducing conditions, transferred onto nitrocellulose membrane, and detected by western blotting with an antibody against an in-frame His tag. Recombinant WT G3 domains migrated at approximately 26 kDa for the short form and 40 kDa for the long form, whereas the mutant proteins migrated more slowly, with a mass of ∼45 kDa for both forms. Protein samples from cell lysates are shown in the left panel and from the culture media in the right panel for each form; the size of molecular weight (MW) markers is shown on the right.
(B) N-glycanase digest of the mutated aggrecan G3 proteins. Western-blot analysis is of purified D2267N G3 protein with (digested) or without (undigested) N-glycanase treatment. The undigested D2267N protein appears as a diffuse ∼45 kDa band corresponding to the glycosylated form, which is in contrast to the digested form that becomes a sharp ∼26 kDa band for the short form and ∼40 kDa for the long form (similar to WT protein), indicating that the secreted mutated proteins possess N-glycan chains.
(C) Binding analysis of recombinant aggrecan G3 domains to tenascin-C. The purity of the recombinant proteins was verified by silver staining of SDS-PAGE gel under reducing conditions (inset). The purified proteins were then tested for their ability to bind tenascin-C. Shown are representative sensograms (surface plasmon resonance, SPR) for WT G3 or D2267N G3 proteins at a concentration of 10 μg/ml. The proteins were injected at a flow rate of 30 μl/min for 3 min.
