Abstract
AIMS
We aimed to examine the frequency of high-dose (defined as mean chlorpromazine mg equivalent doses above 1000) antipsychotic prescriptions in schizophrenia and their clinical correlates in the context of a comparison between studies in 2001 and 2004 within six East Asian countries and territories.
METHODS
Prescriptions of high-dose antipsychotic for a sample of 2136 patients with schizophrenia from six countries and territories (mainland China, Hong Kong, Korea, Japan, Taiwan and Singapore) were evaluated in 2004 and compared with data obtained for 2399 patients in 2001.
RESULTS
Overall, the comparison between 2001 and 2004 showed a significant decrease in high-dose antipsychotic use from 17.9 to 6.5% [odds ratio (OR) 0.32, 95% confidence interval (CI) 0.26, 0.39, P < 0.001]. Patients who received high-dose antipsychotics were significantly more likely to have multiple admissions (OR 1.96, 95% CI 1.16, 3.33, P = 0.009), more positive psychotic symptoms such as delusions (OR 2.05, 95% CI 1.38, 3.05, P < 0.001) and hallucinations (OR 1.85, 95% CI 1.30, 2.64, P = 0.001), but less likely to have negative symptoms (OR 0.58, 95% CI 0.40, 0.82, P = 0.002). Multivariate regression analyses revealed that prescription of high-dose antipsychotics was also predicted by younger age (P < 0.001), time period of study (2001; P < 0.001), use of first-generation antipsychotic (P < 0.001) and depot antipsychotics (P < 0.001) as well as antipsychotic polytherapy (P < 0.001).
CONCLUSIONS
We identified the clinical profile and treatment characteristics of patients who are at risk of receiving high antipsychotic doses. These findings should provide impetus for clinicians to constantly monitor the drug regimes and to foster rational, evidence-based prescribing practices.
Keywords: antipsychotic, high dose, prescriptions, schizophrenia
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
High-dose antipsychotic use in schizophrenia has been a topic of continuous debate since the introduction of the first antipsychotic in the 1950s.
There are reasons arguing for (such as pharmacokinetic reasons for the possible use of high antipsychotic doses in those with genetic polymorphisms associated with unusually high metabolic rates and the presence of inducers such as co-prescriptions of other medications and smoking) and against high-dose antipsychotic use (such as the pharmacokinetic understanding that Asians may need lower antipsychotic doses, the association of high antipsychotic doses with more frequent adverse effects, and receptor occupancy data from neuroimaging studies).
There is increasing awareness of the need for more practice-based research in order to highlight discrepancies between the empirical data and clinical practice.
WHAT THIS STUDY ADDS
There was an overall significant decrease in the frequency of high-dose antipsychotic use from 17.9% in 2001 to 6.5% in 2004 within East Asia.
The association of high antipsychotic doses with demographic, psychopathological and treatment variables identified the clinical profile of schizophrenia patients who are at risk of receiving high antipsychotic doses.
These findings provide information and impetus for clinicians to constantly monitor the drug regimes and to foster rational, evidence-based prescribing practices.
Introduction
The benefit of high-dose antipsychotic use in schizophrenia has been a topic of continuous debate since the introduction of the first antipsychotic in the 1950s [1]. Whereas earlier reports had supported the administration of higher antipsychotic doses in schizophrenia [2, 3], later studies found that the dose–benefit relationships decreased with higher doses [4] and further proposed the use of lowest effective dose in the context of regular monitoring of safety and clinical efficacy parameters [5]. In the PORT guidelines, Lehman et al. [6] recommended that the antipsychotic dose used in the treatment of schizophrenia should remain within the range of 300–1000 mg daily chlorpromazine (CPZ) equivalent doses, prompting pharmacoepidemiological studies to evaluate the prevalence and prescription patterns of high-dose antipsychotic use in different psychiatric settings. Such studies in Europe and the USA have revealed that high-dose antipsychotic use is not an uncommon practice, with rates varying from 15.4 to 41% [7–9]. In comparison, prescription practices regarding high-dose antipsychotic use have been relatively understudied in Asia, particularly in terms of changing trends over time and their clinical correlates, save for three studies. Suzuki et al. [10] found that up to 81% of their study cohort of Japanese patients with chronic schizophrenia received daily antipsychotic doses exceeding 1000 mg mean daily CPZ equivalents. Covering six countries and territories within East Asia, Sim et al. [11] found high-dose antipsychotic prescription in 17.9% of their sample. High antipsychotic doses were associated with certain demographic features (e.g. younger age), psychopathology (e.g. delusions) and other treatment characteristics (e.g. administration of depot medications).
In this study, we set out (i) to examine the frequency of high-dose antipsychotic prescriptions (defined as mean daily CPZ equivalent doses >1000 mg) in patients with schizophrenia, (ii) to evaluate clinical correlates of high-dose antipsychotic use and (iii) to determine changes of prescription trends of high-dose antipsychotics between the two time periods (2001 and 2004) when such studies were conducted. Based on clinical observations, we hypothesized that there was a decrease in the use of high-dose antipsychotics over this time period (2001–2004) in East Asia.
Methods
Study design and participants
The Research on East Asia Psychotropic Prescription (REAP) study is an ongoing pharmacoepidemiological study of real-life prescription trends associated with psychotropic drugs in schizophrenia inpatients within East Asia. The study was initiated in July 2001 and conducted in six East Asian countries and territories (China, Hong Kong, Japan, Korea, Singapore and Taiwan). The details of the REAP study have been described previously [11, 12] and are summarized below.
In July 2001, a cross-sectional study was conducted on a sample of 2399 consecutively admitted inpatients [of whom 55.9% (n = 1340) were male] with schizophrenia in six East Asian countries and territories (China, Hong Kong, Japan, Korea, Singapore and Taiwan) using a standardized protocol. The study with the same design was conducted in July 2004 involving 2136 inpatients [of whom 57.2% (n = 1222) were male] with schizophrenia in the same sites in the six countries and territories. Consensus meetings were held at various sites before the study to discuss issues related to data collection and the uniformity of data entry. Participating patients fulfilled the diagnostic criteria for schizophrenia according to the International Classification of Disease, 10th edn (ICD-10) [13] or the 4th version of the Diagnostic and Statistical Manual of Mental disorders (DSM-IV) [14]. Patients with clinically significant medical conditions or active psychotic symptoms related to comorbid substance use disorders were excluded.
The data collected by psychiatrists were basic sociodemographic information and clinical characteristics including psychopathology and prescription of all psychotropic drugs. Depot antipsychotics given within 30 days of admission were also documented. Daily doses of antipsychotics, including depot preparates, were converted to approximate daily mean chlorpromazine mg equivalents (CPZeq) using standard guidelines ([15–17]; see Table 1). For statistical analysis, the total daily antipsychotic doses were divided into two categories, namely (i) those patients receiving ≤1000 mg day−1 daily mean CPZeq, and (ii) those receiving >1000 mg day−1 CPZeq of antipsychotic medications. The study was approved by the Institutional Research Boards of all the coordinating and funding centres.
Table 1.
Commonly used antipsychotics and their chlorpromazine equivalents (CPZeq) in milligrams per day (mg day−1)*
| Conventional antipsychotic | CPZeq (mg day−1) |
|---|---|
| Chlorpromazine | 100 |
| Haloperidol | 2 |
| Levomepromazine | 100 |
| Sulpiride | 200 |
| Trifluoperazine | 5 |
| Atypical antipsychotic | |
|---|---|
| Clozapine | 50 |
| Olanzapine | 5 |
| Quetiapine | 75 |
| Risperidone | 2 |
| Zotepine | 66 |
Statistical analysis
Antipsychotic doses and relative risks are reported as means ± standard deviation (SD), and odds ratios (OR) with their 95% confidence intervals (CI), respectively. Analyses were performed with the Statistical Package for Social Sciences (SPSS) Version 10.1 (SPSS Inc., Chicago, IL, USA). The normality of distributions of continuous measures was checked with the Kolmogorov–Smirnov one-sample test. Differences between groups (patient with ≤1000 vs. >1000 mg day−1 CPZeq of antipsychotics) were tested by anova (t-test) for normally distributed data, nonparametric Mann–Whitney U-tests for non-normally distributed continuous data, and by contingency tables (x2) for categorical variables. Multiple logistic regression analysis was carried out to adjust for relevant covariates and to determine the predictors of high-dose antipsychotic usage both in 2004 and for 2001 and 2004 as a covariate. Statistical significance required a two-tailed P < 0.05.
Results
Demographic/clinical features and comparison across 2001 and 2004
The basic sociodemographic characteristics of the study population in 2001 and 2004 are shown in Table 2. Overall, in 2004, the mean age (SD) of the study population was 43.09 (14.16) years and the gender distribution was 57.2% male (n = 1222) and 42.8% female (n = 914). Cases of first admission constituted 21.1% (n = 451) of the study sample in 2004. In 2004, high-dose antipsychotics were prescribed in 6.5% (n = 139) of the study sample and the total mean (SD) daily antipsychotic dose was 482.41 (413.78) mg CPZ equivalents. In addition, the total mean (SD) daily antipsychotic doses in 2004 for those on lower and higher antipsychotic doses were 397.43 (240.20) and 1505.16 (637.64) mg CPZ equivalents, respectively.
Table 2.
Demographic data and characteristics of antipsychotic prescription in the six countries and territories in 2001 and 2004
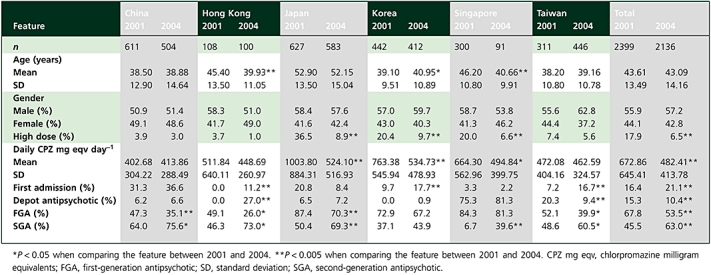 |
Comparing across the 2001 and 2004 samples, there were no significant differences in terms of age and gender. However, for the whole sample, there was a significant decrease in the rates of high-dose antipsychotic use, from 17.9% in 2001 to 6.5% in 2004 (OR 0.32, 95% CI 0.26, 0.39, P < 0.001). In terms of regions, this decrease was significant in Japan (OR 5.88, 95% CI 4.23, 8.16, P < 0.001), Korea (OR 2.38, 95% CI 1.59, 3.55, P < 0.001) and Singapore (OR 3.54, 95% CI 1.48, 8.50, P = 0.002).
The overall mean (SD) daily antipsychotic dose decreased from 672.86 (645.41) mg CPZ equivalents in 2001 to 482.41 (413.78) mg CPZ equivalents by 2004 (t = 10.97, d.f. = 4533, P < 0.001). The decrease was particularly prominent in Japan (t = 10.84, d.f. = 1208, P < 0.001), Korea (t = 6.12, d.f. = 852, P < 0.001) and Singapore (t = 2.27, d.f. = 389, P = 0.024). Overall depot antipsychotic use also decreased from 15.3% in 2001 to 10.4% by 2004 (OR 0.64, 95% CI 0.53, 0.76, P < 0.001). According to the regions, depot antipsychotics were less frequently prescribed in Taiwan (OR 0.41, 95% CI 0.27, 0.62, P < 0.001), in contrast to a significant increase in their use in Hong Kong (OR 1.37, 95% CI 1.22, 1.54, P < 0.001), whereas there was no change in the other sites. When the antipsychotic dosages were divided into quintile distribution of total CPZ mg equivalents (0–200, 200–350, 351–525, 526–833, 833 and above CPZ mg equivalents), the same trend was observed in that higher antipsychotic doses above third quintile were less commonly used in 2004 compared with 2001 (Figure 1). The list of 10 common antipsychotics (including first- and second-generation antipsychotics), their absolute doses and chlorpromazine equivalents across 2001 and 2004 are shown in Table 3.
Figure 1.
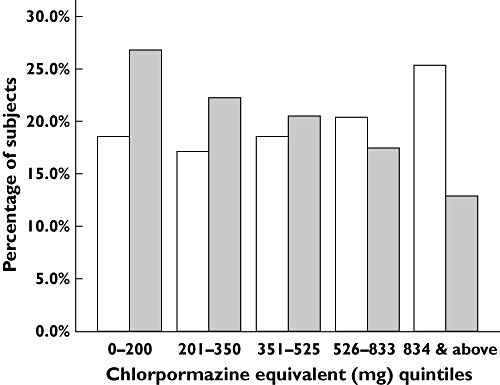
Trends of antipsychotic use in 2001 vs. 2004. Year: 2001(□); 2004 ( )
)
Table 3.
Ten common antipsychotics prescribed across 2001 and 2004
| 2001 (n = 2399) | 2004 (n = 2136) | |||||
|---|---|---|---|---|---|---|
| First-generation antipsychotic | n (%) | Mean doses (SD), mg | CPZ eq (SD), mg day−1 | n (%) | Mean doses (SD), mg | CPZ eq (SD), mg day−1 |
| Haloperidol | 691 (28.8) | 15.9 (11.8) | 793.9 (587.7) | 387 (18.1) | 14.3 (10.3) | 713.5 (515.0) |
| Chlorpromazine | 561 (23.4) | 231.0 (201.3) | 231.0 (201.3) | 349 (16.3) | 213.8 (204.9) | 213.8 (204.9) |
| Levomepromazine | 247 (10.3) | 77.5 (90.9) | 77.5 (90.9) | 165 (7.7) | 83.9 (85.7) | 83.9 (85.7) |
| Sulpiride | 233 (9.7) | 606.4 (370.4) | 303.2 (185.2) | 178 (8.3) | 656.7 (411.2) | 328.4 (205.6) |
| Trifluoperazine | 115 (4.8) | 17.4 (10.1) | 348.1 (201.3) | 41 (1.9) | 13.5 (10.5) | 270.0 (210.0) |
| Second-generation antipsychotic | ||||||
| Risperidone | 473 (19.7) | 4.7 (2.6) | 235.0 (130.0) | 631 (29.5) | 4.7 (2.4) | 235.0 (120.0) |
| Clozapine | 348 (14.5) | 130.1 (77.7) | 260.2 (155.3) | 340 (15.9) | 120.1 (60.3) | 240.2 (120.6) |
| Zotepine | 137 (5.7) | 181.7 (115.3) | 272.5 (173.3) | 10 (0.5) | 112.0 (31.6) | 168.0 (47.4) |
| Olanzapine | 115 (4.8) | 16.3 (6.0) | 326.0 (120.0) | 226 (10.6) | 15.3 (6.1) | 306.0 (122.0) |
| Quetiapine | 86 (3.6) | 444.8 (251.5) | 578.3 (326.9) | 168 (7.9) | 420.3 (250.0) | 546.4 (325.0) |
CPZ eq, chlorpromazine equivalents; SD, standard deviation.
Clinical correlates of high-dose antipsychotic use in 2004
There was no difference between patients receiving and not receiving high-dose antipsychotic medications with respect to age and gender (Table 4). Patients who received high-dose antipsychotics were less likely to be first admissions, more likely to have positive psychotic symptoms and less likely to have negative symptoms. Patients with aggressive behaviour were likely to be in receipt of high antipsychotic doses. With regard to the type of antipsychotics prescribed, patients on high-dose antipsychotics were more likely to be given first-generation and less likely to receive second-generation antipsychotics. They were also more likely to be on more than one antipsychotic drug and complain of constipation.
Table 4.
Comparison of patient receiving ≤1000 mg day−1 CPZ eq vs. >1000 mg day−1 CPZ eq of antipsychotic drugs in the whole sample in 2004
| Feature | ≤1000 mg (n = 1997) | >1000 mg (n = 139) | Test statistica | P |
|---|---|---|---|---|
| Mean age (SD), years | 43.15 (14.27) | 42.14 (12.44) | – | NS |
| OR (95% CI) | ||||
| Gender | – | NS | ||
| % male | 93.9 | 6.1 | ||
| % female | 93.0 | 7.0 | ||
| First admission (%) | 96.2 | 3.8 | 0.51 (0.30, 0.86) | 0.009 |
| Delusion (%) | 91.9 | 8.1 | 2.05 (1.38, 3.05) | <0.001 |
| Hallucination (%) | 91.6 | 8.4 | 1.85 (1.30, 2.64) | 0.001 |
| Disorganized speech (%) | 91.3 | 8.7 | 1.58 (1.10, 2.27) | 0.017 |
| Negative symptoms (%) | 94.9 | 5.1 | 0.58 (0.40, 0.82) | 0.002 |
| Physical aggression (%) | 88.7 | 11.3 | 2.05 (1.32, 3.17) | 0.002 |
| Depot antipsychotic (%) | 93.7 | 6.3 | – | NS |
| First-generation antipsychotic (%) | 90.4 | 9.6 | 3.55 (2.33, 5.39) | <0.001 |
| Antipsychotic polytherapy (%) | 93.2 | 6.8 | 1.07 (1.06, 1.09) | 0.009 |
| Second-generation antipsychotic (%) | 94.6 | 5.4 | 0.63 (0.45, 0.89) | 0.011 |
| Constipation (%) | 90.5 | 9.5 | 1.95 (1.38, 2.76) | <0.001 |
Comparisons are based on bivariate analyses. CI, confidence interval; CPZ mg eqv, chlorpromazine milligram equivalents; OR, odds ratio; SD, standard deviation.
Factors associated with high-dose antipsychotic use
Using multivariate regression analyses, in 2004 high-dose antipsychotic use was associated with younger age, multiple admissions to hospital, presence of delusions as well as the prescription of first-generation antipsychotics in 2004 (Table 5). Inclusion of 2001 and 2004 within the analyses revealed that prescription of high-dose antipsychotics was further predicted by study region, the time of study (2001), use of depot and more than one antipsychotic in the treatment regime (Table 6).
Table 5.
Factors associated with high-dose antipsychotic use in the whole sample in 2004
| B | SE | Wald | P | OR* | 95.0% CI | |
|---|---|---|---|---|---|---|
| Age | −0.02 | 0.01 | 4.25 | 0.04 | 0.98 | 0.97, 0.99 |
| Gender | 0.20 | 0.19 | 1.14 | NS | 1.22 | 0.85, 1.76 |
| First admission | 0.68 | 0.28 | 5.89 | 0.015 | 0.51 | 0.29, 0.88 |
| Delusion | 0.41 | 0.22 | 3.32 | NS | 1.50 | 0.97, 2.33 |
| Hallucination | 0.34 | 0.20 | 2.89 | NS | 1.41 | 0.95, 2.08 |
| Disorganized speech | 0.26 | 0.20 | 1.70 | NS | 1.29 | 0.88, 1.90 |
| Negative symptoms | −0.27 | 0.20 | 1.83 | NS | 0.77 | 0.52, 1.13 |
| Aggression (physical) | 0.37 | 0.25 | 2.22 | NS | 1.44 | 0.89, 2.33 |
| Depot antipsychotic | −0.06 | 0.36 | 0.03 | NS | 0.94 | 0.46, 1.90 |
| FGA | 1.07 | 0.27 | 15.28 | <0.001 | 2.92 | 1.71, 5.01 |
| SGA | −0.08 | 0.23 | 0.12 | NS | 0.92 | 0.59, 1.45 |
| Antipsychotic polytherapy | 4.59 | 6.61 | 0.48 | NS | 98.29 | 0.00, 200.45 |
Analysis is based on multivariate logistic regression modelling. CI, confidence interval; FGA, first-generation antipsychotic; OR, odds ratio; SGA, second-generation antipsychotic.
Table 6.
Predictors of high-dose antipsychotic use (including two samples from 2001 and 2004)
| Factor | B | SE | Wald | P | OR* | 95.0% CI |
|---|---|---|---|---|---|---|
| Country (China) | −0.99 | 0.26 | 14.92 | <0.001 | 0.37 | 0.22, 0.61 |
| Country (Japan) | 0.71 | 0.21 | 21.78 | 0.001 | 2.04 | 1.36, 3.06 |
| Country (Korea) | 0.60 | 0.20 | 8.52 | 0.004 | 1.81 | 1.22, 2.71 |
| Age | −0.02 | 0.01 | 18.56 | <0.001 | 0.98 | 0.97, 0.98 |
| Gender | 0.15 | 0.11 | 1.95 | NS | – | – |
| First admission | 0.11 | 0.15 | 0.51 | NS | – | – |
| Depot antipsychotic use | 1.21 | 0.19 | 42.72 | <0.001 | 3.36 | 2.34, 4.84 |
| FGA use | 1.09 | 0.20 | 30.24 | <0.001 | 2.96 | 2.01, 4.36 |
| SGA use | −0.02 | 0.13 | 0.03 | NS | – | – |
| Period of study 2001 | −1.94 | 0.13 | 239.51 | <0.001 | 8.77 | 5.40, 8.85 |
| Antipsychotic polytherapy | 2.21 | 0.17 | 178.65 | <0.001 | 9.13 | 6.60, 12.63 |
Analysis is based on multivariate logistic regression modelling, pooling data for 2001 and 2004. CI, confidence interval; FGA, first-generation antipsychotic; OR, odds ratio; SGA, second-generation antipsychotic.
Discussion
There are several findings from this study. First, there was an overall significant decrease in the frequency of high-dose antipsychotic use from 2001 to 2004, confirming our hypothesis that was based on clinical impressions. Second, in 2004 high-dose antipsychotic use was associated with clinical (multiple admissions, positive psychotic symptoms, physical aggression) and treatment variables (more first-generation and less second-generation antipsychotics and antipsychotic polytherapy). Third, using multivariate regression modelling in the overall sample and pooling data from 2001 and 2004, significant associations of high-dose antipsychotic prescription included younger age, region and time of study, use of depot and first-generation antipsychotics and antipsychotic polytherapy.
The frequency of high-dose antipsychotic use (6.5% in 2004), although significantly lower than in 2001, highlighted the fact that this is not necessarily an uncommon practice in East Asia. However, notably, this rate is lower than the findings of most European and American studies, which range from 15.4 to 41% [7]. Barbui et al. [7] found that high-dose antipsychotics were prescribed to 15.4% of their study population in Italy, which comprised all psychiatric inpatients, including those with diagnosis of schizophrenia spectrum disorders. In another study involving four European sites (Croydon, Leipzig, Amsterdam and Verona), up to 28% of patients with schizophrenia received high doses of antipsychotics [18]. Similarly, Diaz et al. [8] found that between 27 and 41% of schizophrenia patients in state psychiatric hospitals received high antipsychotic doses. Comparison of the frequency of high-dose antipsychotic prescriptions across studies needs to take certain factors into consideration, including the type of patients (schizophrenia vs. inpatients with other psychiatric diagnoses), definition of what constitutes a high-dose antipsychotic (e.g. PORT guidelines vs. definition by the World Health Organization) [6, 19] as well as the different study sites with their general socio-cultural and specific psychiatric traditions and economic situation as major factors determining antipsychotic prescriptions.
Our earlier study had documented wide variations of high-dose antipsychotic prescription practices with respect to countries within East Asia, varying from 3.7% in Hong Kong to a maximum of 36.5% in Japan [11]. In comparison, this range narrowed by 2004, with rates varying from 1% in Hong Kong to a maximum of 9.7% in Korea. There are reasons arguing against high-dose antipsychotic use. These arguments include the pharmacokinetic understanding that Asians may need lower antipsychotic doses [20], the association of high antipsychotic doses with more frequent adverse effects [21], worsening cognitive function [22], and higher mortality [23]. In addition, the lack of evidence for administering high antipsychotic doses has come from positron emission tomography neuroimaging studies [24–26] and was further suggested by their negative impact on patient's adherence with these drugs [27]. However, recent data from pharmacological and clinical studies have suggested a possible rationale for the use of higher antipsychotic doses in a subset of schizophrenia patients. In the CATIE study [28], olanzapine given at >50% upper limit was superior to lower doses in several parameters, including lower frequencies and longer time of all cause discontinuation, discontinuation due to lack of efficacy and patients’ decision, shorter duration for hospitalization due to illness exacerbation, and longer duration of effective treatment. The CATIE findings suggest that certain antipsychotic doses at higher than those recommended may be beneficial in certain cases at some point in the course of the illness [29]. Higher antipsychotic doses may be needed in more severe illness and in manifestations of hostility [26], such as the association with physical aggression as found in this study. Botts et al. [30] also proposed pharmacokinetic reasons for the possible use of high antipsychotic doses such as in those with genetic polymorphism variations associated with unusually high metabolic rates and the presence of inducers such as co-prescriptions of other medications and smoking.
The association of high antipsychotic doses with younger age in this study is consistent with the findings of other studies [8, 9]. Valenstein et al. [9] surveyed the prescription patterns for 936 veterans with schizophrenia across 14 facilities and found that high antipsychotic doses were associated with the age range of 21–44 years. Similarly, Diaz et al. [8] reported that high antipsychotic doses were more prevalent under the age of 56 years. The lack of impact of gender on prescribing high-dose antipsychotics in this study is in contrast with that of other investigations, which found such an association with either male [7] or female schizophrenia patients [31].
High antipsychotic doses were more likely to be associated with delusions and hallucinations, but not negative symptoms in this study. These findings confirm those of earlier studies that reported similar relationships with positive psychotic symptoms, but not negative symptoms [7]. Barbui et al. [7] noted that high antipsychotic doses were related to a greater severity of psychopathology. The severity of symptoms frequently persists even at 3 months after the patients’ discharge from hospital [32]. This behoves clinicians to monitor the clinical status of their patients regularly and to re-evaluate the basis for the continued use of high antipsychotic doses.
In terms of the characteristics of the treatment regime, prescription of high antipsychotic doses was associated with that of first-generation antipsychotics including depot antipsychotics as well as less use of second-generation antipsychotics that could possibly be related to the limited availability of depot preparations of second-generation antipsychotics. Depot antipsychotic administration increased the odds of high antipsychotic dose by 30 in 293 schizophrenia inpatients from four New York hospitals [33]. More recently, Barbui et al. [17] in their study of 375 patients with schizophrenia in Europe found that persistence of high antipsychotic doses was associated with the concurrent use of first- and second-generation antipsychotics at baseline, replicating earlier observations [34, 35] that antipsychotic polytherapy is also a risk factor for high-dose antipsychotic use. Our study has confirmed that antipsychotic polytherapy is associated with high antipsychotic doses in our study population.
There was a significant decrease of high-dose antipsychotic prescriptions between 2001 and 2004 in Japan, Korea and Singapore, in addition to the use of lower total daily antipsychotic doses during the same period. Several reasons may be posited for these changes. First, involvement of the clinicians in the two studies may have an impact on the prescription practices in their settings. Second, the publicity generated by the REAP studies through local scientific symposia, presentations at international conferences and journal publications may have enhanced clinicians’ familiarity with rational prescription practices [36]. Third, more frequent use of second-generation antipsychotics, made possible by increased healthcare funding for these medications, might lower the total daily antipsychotic doses, leading to the decrease in overall high-dose antipsychotic prescriptions. Strategies to enhance rational prescribing patterns include peer-supported educational programmes [37], careful evaluation and monitoring of local practices, consideration of dose reduction [10] or dose optimization in appropriate cases [38], repeated comparative examinations of local and international prescription practices over time and conveying the findings to clinicians [39].
There were a few limitations to this study. First, the lack of use of standardized rating instruments did not allow the quantification of the severity of psychopathology or the adverse effects. Second, the heterogeneity of the healthcare systems and the different focus on hospitalization in the different sites might also limit the generalizability of these findings to other healthcare settings, including patient populations with schizophrenia that are managed in the community. Third, other factors influencing prescription practices, such as structure of the healthcare systems, funding mechanisms, past experiences and perceptions of clinicians towards high-dose antipsychotic use, were not evaluated.
Despite the above limitations, to the best of our knowledge this is the first study to examine changes in prescription practices of antipsychotics and report a significant decrease of high-dose antipsychotic use over time and their clinical correlates in East Asia. The association of high antipsychotic doses with demographic, clinical and treatment variables allows a better appreciation of patients who are at risk of receiving high antipsychotic doses, their clinical profiles as well as the characteristics of their treatment regimes. This should provide the impetus for clinicians to constantly monitor drug regimes, keep abreast of new developments in the literature and strive for rational prescribing practices in the context of real clinical situations and evidence-based medicine.
Acknowledgments
Supported by research funds from (i) Institute of Mental Health, Department of Research, Singapore (K.S.), (ii) International Centre for Medical Research, Japan (S.F., N.S.), (iii) Bureau of National Health Insurance, Taiwan (S.Y., M.Y.C.).
Competing interests
NS served as a consultant and/or participant in scientific meetings organised by Eli Lilly, Pfizer, Servier and Lundbeck.
REFERENCES
- 1.Ayd FJ. Large doses of chlorpromazine in the treatment of psychiatric patients. Dis Nerv Syst. 1955;16:146–9. [PubMed] [Google Scholar]
- 2.Prien RF, Cole JO. High dose chlorpromazine therapy in chronic schizophrenia. Report of National Institute of Mental Health – psychopharmacology research branch collaborative study group. Arch Gen Psychiatry. 1968;18:482–95. [PubMed] [Google Scholar]
- 3.McClelland HA, Farquharson RG, Leyburn P, Furness JA, Schiff AA. Very high dose fluphenazine decanoate: a controlled trial in chronic schizophrenia. Arch Gen Psychiatry. 1976;33:1435–9. doi: 10.1001/archpsyc.1976.01770120039003. [DOI] [PubMed] [Google Scholar]
- 4.Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry. 1988;45:79–915. doi: 10.1001/archpsyc.1988.01800250095013. [DOI] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Mental Health. London: National Institute of Clinical Excellence Guidelines; Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. [Google Scholar]
- 6.Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- 7.Barbui C, Biancosino B, Esposito E, Marmai L, Dona S, Grassi L. Factors associated with antipsychotic dosing in psychiatric inpatients: a prospective study. Int Clin Psychopharmacol. 2007;22:221–5. doi: 10.1097/YIC.0b013e3281084ea8. [DOI] [PubMed] [Google Scholar]
- 8.Diaz FJ, De Leon J. Excessive antipsychotic dosing in 2 U.S. State hospitals. J Clin Psychiatry. 2002;63:998–1003. doi: 10.4088/jcp.v63n1107. [DOI] [PubMed] [Google Scholar]
- 9.Valenstein M, Copeland L, Owen R, Blow F, Visnic S. Delays in adopting evidence-based dosages of conventional antipsychotics. Psychiatr Serv. 2001;52:1242–4. doi: 10.1176/appi.ps.52.9.1242. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Uchida H, Tanaka KF, Tomita M, Tsunoda K, Nomura K, Takano H, Tanabe A, Watanabe K, Yagi G, Kashima H. Reducing the dose of antipsychotic medications for those who had been treated with high-dose antipsychotic polypharmacy: an open study of dose reduction for chronic schizophrenia. Int Clin Psychopharmacol. 2003;18:323–9. doi: 10.1097/00004850-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Sim K, Su A, Leong JY, Yip K, Chong MY, Fujii S, Yang S, Ungvari GS, Si T, Chung EK, Tsang HY, Shinfuku N, Kua EH, Tan CH. High dose antipsychotic use in schizophrenia: findings of the REAP (research on east Asia psychotropic prescriptions) study. Pharmacopsychiatry. 2004;37:175–9. doi: 10.1055/s-2004-827174. [DOI] [PubMed] [Google Scholar]
- 12.Sim K, Su A, Fujii S, Yang SY, Chong MY, Ungvari GS, Si T, Chung EK, Tsang HY, Chan YH, Heckers S, Shinfuku N, Tan CH. Antipsychotic polypharmacy in patients with schizophrenia: a multicentre comparative study in East Asia. Br J Clin Pharmacol. 2004;58:178–83. doi: 10.1111/j.1365-2125.2004.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organisation. International Statistical Classification of Diseases and Related Health Problems. Geneva: World Health Organisation; 1992. 10 rev. [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 15.American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Schizophrenia. Washington DC: American Psychiatric Press; 2004. [Google Scholar]
- 16.Kane JM, Aguglia E, Altamura AC, Ayuso Gutierrez JL, Brunello N, Fleischhacker WW, Gaebel W, Gerlach J, Guelfi JD, Kissling W, Lapierre YD, Lindström E, Mendlewicz J, Racagni G, Carulla LS, Schooler NR. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Eur Neuropsychopharmacol. 1998;8:55–66. doi: 10.1016/s0924-977x(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 17.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 18.Barbui C, Nose M, Mazzi MA, Thornicroft G, Schene A, Becker T, Bindman J, Leese M, Helm H, Koeter M, Weinmann S, Tansella M. Persistence with polypharmacy and excessive dosing in patients with schizophrenia treated in four European countries. Int Clin Psychopharmacol. 2006;21:355–62. doi: 10.1097/01.yic.0000224785.68040.43. [DOI] [PubMed] [Google Scholar]
- 19.WHO Collaborating Centre for Drug Statistic Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo: WHO Collaborating Centre for Drug Statistic Methodology, World Health Organisation; 2003. [Google Scholar]
- 20.Ungvari GS, Pang AH, Chiu HF, Wong CK, Lum FC. Psychotropic drug prescription in rehabilitation. A survey in Hong Kong. Soc Psychiatry Psychiatr Epidemiol. 1996;31:288–91. doi: 10.1007/BF00787922. [DOI] [PubMed] [Google Scholar]
- 21.Carnahan RM, Lund BC, Perry PJ, Chrischilles EA. Increased risk of extrapyramidal side-effect treatment associated with atypical antipsychotic polytherapy. Acta Psychiatr Scand. 2006;113:135–41. doi: 10.1111/j.1600-0447.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 22.Krausz M, Moritz S, Lambert M, Naber D. Dosage of conventional neuroleptic medication and subjective cognitive functioning in schizophrenia. Int Clin Psychopharmacol. 2000;15:77–81. doi: 10.1097/00004850-200015020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Waddington JL, Youssef HA, Kinsella A. Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry. 1998;173:325–9. doi: 10.1192/bjp.173.4.325. [DOI] [PubMed] [Google Scholar]
- 24.Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. Central D2 dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;15:227–35. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 25.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 26.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 27.McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia. A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry. 1991;48:739–45. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 29.Correll CU. Real-life dosing with second-generation antipsychotics. J Clin Psychiatry. 2005;66:1610–1. doi: 10.4088/jcp.v66n1218. [DOI] [PubMed] [Google Scholar]
- 30.Botts S, Littrell R, de Leon J. Variables associated with high olanzapine dosing in a state hospital. J Clin Psychiatry. 2004;65:1138–43. doi: 10.4088/jcp.v65n0817. [DOI] [PubMed] [Google Scholar]
- 31.Zito JM, Craig TJ, Wanderling J, Siegel C. Pharmaco-epidemiology in 136 hospitalized schizophrenic patients. Am J Psychiatry. 1987;144:778–82. doi: 10.1176/ajp.144.6.778. [DOI] [PubMed] [Google Scholar]
- 32.Sohler NL, Walkup J, McAlpine D, Boyer C, Olfson M. Antipsychotic dosage at hospital discharge and outcomes among persons with schizophrenia. Psychiatr Serv. 2003;54:1258–63. doi: 10.1176/appi.ps.54.9.1258. [DOI] [PubMed] [Google Scholar]
- 33.Walkup JT, McAlpine DD, Olfson M, Labay LE, Boyer C, Hansell S. Patients with schizophrenia at risk for excessive antipsychotic dosing. J Clin Psychiatry. 2000;61:344–8. doi: 10.4088/jcp.v61n0504. [DOI] [PubMed] [Google Scholar]
- 34.Centorrino F, Goren JL, Hennen J, Salvatore P, Kelleher JP, Baldessarini RJ. Multiple versus single antipsychotic agents for hospitalized psychiatric patients: case–control study of risks versus benefits. Am J Psychiatry. 2004;161:700–6. doi: 10.1176/appi.ajp.161.4.700. [DOI] [PubMed] [Google Scholar]
- 35.Tognomi G. Pharmacoepidemiology of psychotropic drugs in patients with severe mental disorders in Italy. Italian Collaborative Study Group on the Outcome of Severe Mental Disorders. Eur J Clin Pharmacol. 1999;55:685–90. doi: 10.1007/s002280050694. [DOI] [PubMed] [Google Scholar]
- 36.Knottnerus JA, Dinant GJ. Medicine based evidence, a prerequisite for evidence based medicine. BMJ. 1997;315:1109–10. doi: 10.1136/bmj.315.7116.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Covell NH, Jackson CT, Evans AC, Essock SM. Antipsychotic prescribing practices in Connecticut's public mental health system: rates of changing medications and prescribing styles. Schizophr Bull. 2002;28:17–29. doi: 10.1093/oxfordjournals.schbul.a006920. [DOI] [PubMed] [Google Scholar]
- 38.Volavka J, Cooper TB, Czobor P, Lindenmayer JP, Citrome LL, Mohr P, Bark N. High-dose treatment with haloperidol: the effect of dose reduction. J Clin Psychopharmacol. 2000;20:252–6. doi: 10.1097/00004714-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Ungvari GS, Chung YG, Chee YK, Fung-Shing N, Kwong TW, Chiu HF. The pharmacological treatment of schizophrenia in Chinese patients: a comparison of prescription patterns between 1996 and 1999. Br J Clin Pharmacol. 2002;54:437–44. doi: 10.1046/j.1365-2125.2002.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


