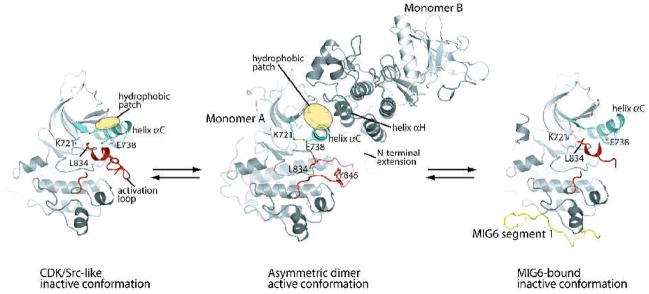
Figure 1.
Autoinhibition, activation and feedback inhibition of the EGFR kinase domain. The EGFR kinase domain is autoinhibited by adopting the CDK/Src-like inactive conformation (left panel) [26]. The N-terminal portion of the activation loop (red) forms a short helix which packs against the N-lobe of the kinase and stabilizes the outwardly displaced conformation of helix αC (aqua). Upon ligand induced dimerization of the receptor, the kinase domain forms the asymmetric activating dimer (middle panel) [26]. In this dimer, monomer B activates monomer A by acting as a cyclin-like activator. The hydrophobic patch in the N-lobe of monomer A (yellow ellipse) is buried in the dimer interface. MIG6 is upregulated immediately after EGFR activation and serves as a feedback inhibitor. MIG6 segment 1 (yellow) binds the C-lobe face of the asymmetric dimer interface, sterically hindering the formation of the asymmetric dimer (right panel) [27].