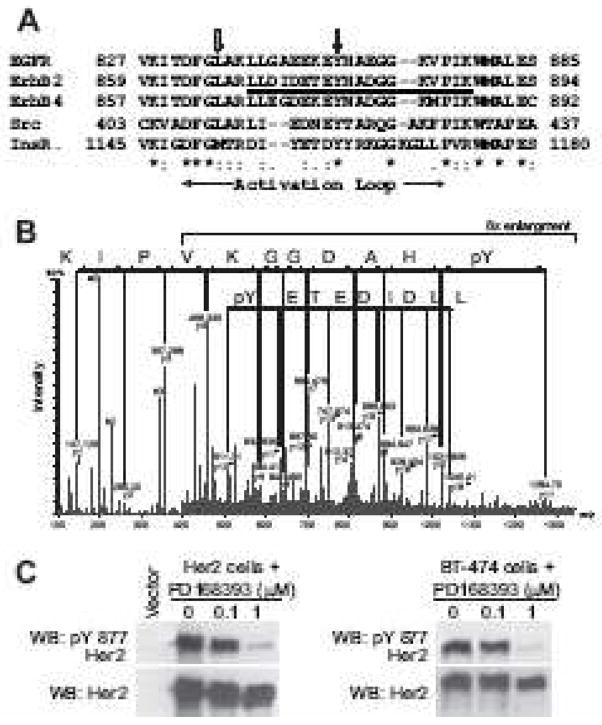
Figure 2.
Identification of Tyr877 phosphorylation site in ErbB2 kinase domain from [49]. (A) Multiple sequence alignment of the kinase domains of human EGFR, ErbB2, ErbB4, Src, and insulin receptor. The phosphopeptide identified by MS in (B) is underlined. Black and gray arrows mark the conserved tyrosine residue and EGFR Leu834, respectively. The activation loop is indicated by horizontal arrows. (B) MS/MS spectra of the peptide, LLDIDETE(pY)HADGGKVPIK, bearing the phosphotyrosine residue at Tyr877 of ErbB2. The charge state of the parent ion is +3, thus yielding both +1(upper line) and +2 charged daughter ions (lower line) on fragmentation. The mass difference corresponding to the phosphotyrosine residue, y11 minus y10 ion, is seen in both +1 and +2 charged fragment series. (C) ErbB2-transfected NIH3T3 fibroblasts or BT-474 breast cancer cells which overexpress endogenous ErbB2 were treated with the ErbB2 inhibitor, PD168393, for 1 hour and immunoblotting performed with phospho-Tyr877 specific ErbB2 antibody or anti-total ErbB2 antibody.