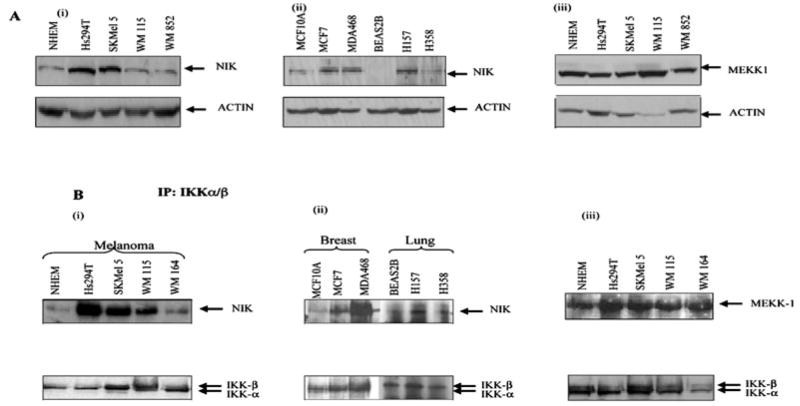
Fig. 3. The level of NIK protein and IKK-associated NIK is higher in some melanoma cells.
A, the normal cells, melanoma, breast cancer, and lung cancer cell lines were incubated in serum-free media overnight. The proteins were resolved on a 10% SDS-PAGE under reducing conditions and transferred to nitrocellulose membrane. The membrane was blotted with NIK and MEKK1 antibody and was stripped and reprobed with actin antibody to monitor equal loading of protein. This figure is representative of three separate experiments. B, co-immunoprecipitation assays. Whole cell extracts were made from normal and malignant cells (melanoma, breast, and lung cancer), and then 800 μg of protein from each sample was used for immunoprecipitation using IKKα and -β antibody (2 μg) to monitor the endogenous levels. These immunoprecipitated extracts were then used to monitor the NIK or MEKK1 associated with IKKαβ by Western blot as described under “Materials and Methods.” The same membrane was blotted with IKKαβ for normalization. This figure is a representative of three different experiments.