Abstract
Diagnosing catheter-related bloodstream infection (CRBSI) still often involves tip culture. The conventional method is the semiquantitative roll plate method. However, the use of a quantitative sonication technique could have additional value, as it may detect endoluminal microorganisms more easily. Because endoluminal infection tends to occur in long-term central venous catheters, we compared both techniques for patients with long-term tunnelled catheters. For 313 consecutive Hickman catheter tips from 279 hematological patients, colonization detection rates were compared by performing both techniques in a random order, using conventional detection cutoffs. Additionally, for the subgroup of patients with clinical suspicion of CRBSI (n = 89), the diagnostic values of both techniques were compared. The overall tip colonization rate was 25%. For each technique, the detection rate tended to be better if that technique was performed first. The diagnostic performance for the subgroup of patients with clinical suspicion of CRBSI was limited and not different for both methods. Sensitivity and specificity were 45% and 84%, respectively, for sonication versus 35% and 90%, respectively, for the roll plate technique. The fact that 35 of 40 patients with CRBSI received antimicrobial therapy before catheter removal and tip culture, in an attempt to salvage the catheter, may partly explain this poor performance. No differences were observed when catheters were stratified according to in situ time below or above the median of 4 weeks. The sonication culture technique was not better than the roll plate method to diagnose tip colonization or CRBSI in patients with long-term tunnelled catheters.
Central venous catheter (CVC)-related bloodstream infection (CRBSI) remains one of the leading causes of nosocomially acquired bacteremia, with significant contributions to morbidity, costs, and to a lesser extent, also mortality (7, 8, 10, 17, 27). Although ideally the diagnosis of CRBSI is made before catheter removal, a definite diagnosis still often involves a culture from the catheter tip (16, 22). The reference standard of tip culture is a semiquantitative technique described by Maki et al. in 1977 (14), with a cutoff of 15 CFU to distinguish microbial contamination of catheters from significant colonization. This technique is also called the roll plate method, as the catheter tip is rolled back and forth on an agar plate for culture. However, because catheter colonization and infection can be the consequence of the introduction of intraluminal microorganisms during manipulation of the catheter hubs or the infusion of fluids or drugs, this technique may be less appropriate for the detection of endoluminal tip colonization.
Other methods were developed subsequently, in an attempt to deal with this and other limitations (1, 4, 5, 9, 11, 12, 24). One promising method with the claimed ability to detect both endoluminal and exoluminal microorganisms is the quantitative sonication technique, first described by Constantinou et al. (6) and validated later. In early years, most studies were performed using cutoffs for catheter tip colonization of ≥1,000 CFU/catheter segment for quantitative techniques, although lower breakpoints were also used (25). Nowadays, laboratory criteria usually accept a breakpoint of ≥100 CFU/catheter segment, which is also recommended in current Infectious Diseases Society of America guidelines (16, 23). Recently, Bouza and colleagues demonstrated a cutoff of ≥100 CFU to be superior to one of ≥1,000 CFU/catheter segment (3).
The number of prospective studies that compare the semiquantitative and quantitative techniques, however, is limited (3, 11, 13, 18, 23, 28). These studies report conflicting results and are nonhomogenous with respect to the type of catheter studied or the length of time that devices remained in place, two important factors that determine the risk of CRBSI (8, 15). Because endoluminal contamination is thought to be the most frequent route of microbial colonization in patients with catheters with a long dwell time, quantitative methods may be especially appropriate for this patient category (15, 16, 19, 22). However, no comparative prospective studies have been performed with this subgroup, and no gold standard exists.
The present study describes the results of a prospective, randomized study to compare the yields of both techniques to detect catheter tip colonization in patients with long-term tunnelled catheters. Tip colonization is a relevant end point, as the incidence of tip colonization was demonstrated to correlate well with the incidence of CRBSI in a recent meta-analysis (21). We also assessed whether performing tip culture, especially by sonication, for patients with clinical suspicion of CRBSI could give additional diagnostic information to rule out or establish CRBSI.
MATERIALS AND METHODS
Patient population.
The study was performed at Erasmus MC, a university referral hospital in Rotterdam, The Netherlands. All consecutive tunnelled Hickman catheters were derived from adult patients with hematological disease, mainly patients with acute myeloid leukemia or myelodysplastic syndrome, with some of them treated with allogeneic stem cell transplantation and invariably with prolonged neutropenia due to the antileukemic treatment. Catheters removed for any reason (e.g., end of therapy or suspicion of CRBSI) in the period from April 2005 until December 2007 were sent to the microbiology department for tip culture. No antimicrobial-coated catheters were used, and connection to the infusion system was established through the use of a needle-free closed connector valve system (Bionector, Valley Forge, PA). All catheters had been placed in the radiology room under full sterile barrier precautions and were mainly used for the administration of intravenous medication and chemotherapy. Data were collected on the use of antimicrobials before catheter removal, catheter dwell time, and reason for removal.
Microbiological procedures.
Catheter tips were processed by using both Maki et al.'s technique and sonication in a random order. Randomization occurred at the microbiology laboratory. The semiquantitative method of Maki et al. was performed by rolling the external surface of a catheter tip back and forth on the surface of a Columbia agar plate supplemented with 5% sheep blood (BD, Franklin Lakes, NJ) at least three times and then incubating the plate for 72 h at 5% CO2 and 35°C, after which the number of CFU were quantitated as described in detail elsewhere (14). Sonication was performed by placing the catheter in 5 ml of 0.9% NaCl, sonicating it for 1 minute (Soniprep 150 instrument with a 23-kHz generator; MSE Ltd., London, United Kingdom), and vortexing it for 15 s. Fifty microliters of the sonication fluid was cultured on Columbia agar, allowing for a detection limit of ≥100 CFU/catheter tip. Finally, the tip was incubated in tryptic soy agar broth. If growth of 1 to 3 CFU was observed on the agar plate on which the sonication fluid had been inoculated, the identification of these colonies was confirmed by broth culture of the tip to exclude contamination of the plate. Microorganisms recovered from the plates were identified and counted by standard microbiological methods. Blood cultures were processed according to routine procedures, using the Bactec system (BD, Franklin Lakes, NJ).
Definitions.
Catheter tip colonization was defined as a positive semiquantitative tip culture of ≥15 CFU/ml for the roll plate method or ≥100 CFU/catheter segment for the sonication technique, as described elsewhere (3, 14, 23, 24).
Definitions of CRBSI and catheter colonization from current guidelines were followed (2, 16, 20). CRBSI was defined as one or more positive blood cultures (at least two blood cultures for coagulase-negative staphylococci) obtained from a peripheral vein for patients with clinical manifestations of infection and no apparent other source of infection except for the catheter and a catheter tip culture with the same phenotypic microorganisms or a differential time to positivity (DTTP) of ≥2 h for the peripheral versus the CVC blood culture. Endoluminal CRBSI was defined as a positive hub culture and a DTTP of ≥2 h or a positive hub culture with the presence of the same microorganism both in peripheral blood and on the catheter tip in the absence of exit site infection and in the absence of any other source of infection. Exoluminal CRBSI was defined as clinical signs of an exit site or tunnel infection combined with a negative hub culture, but with either a DTTP of ≥2 h or positive blood and catheter tip cultures for the same phenotypic microorganism.
Data analysis.
The presence of significant counts of microorganisms assessed by any of the two techniques, using the cutoff values described above, was considered the reference standard for detection. Proportions of detection of tip colonization were calculated for both techniques, taking into account the randomly assigned order.
For the subgroup of patients with clinical suspicion of CRBSI and/or exit site infection with concomitant bacteremia, the sensitivity, specificity, and predictive values with corresponding 95% confidence intervals (Wilson score interval method with continuity correction) were calculated for both techniques separately and combining the results of the two culture methods. In a further exploratory analysis, catheters were stratified according to dwell time (below or above the median dwell time), and sensitivity, specificity, and predictive values were calculated for both techniques. Statistical analyses were performed using SPSS, version 13.0 (Chicago, IL), and VassarStats (New York, NY).
RESULTS
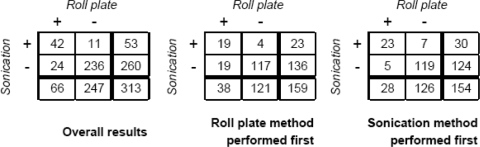
A total of 313 catheter tips from 279 patients were analyzed. The mean dwell time was 55 days (range, 4 to 469 days). Colonization was detected in 77 of 313 catheter tips (25%). Data are presented in Fig. 1. Data were also analyzed with catheters stratified according to the procedure order. For 159 tips, the roll plate method was performed first, whereas for the other 154 catheter tips sonication was performed first. In the sample in which the roll plate method was performed first, tip culture was positive in 38 of 159 cases (24%) with the roll plate method and in 23 of 159 cases (14%) with sonication. In the sample of 154 catheters in which sonication was performed first, the tip culture was positive in 30 cases (19%) detected by sonication and in 28 cases (18%) detected by the roll plate method.
FIG. 1.
Detection of catheter tip colonization in 313 tunneled catheters. Data are presented as overall numbers together with results stratified according to procedure order. Cutoffs used for detection of colonization were ≥15 CFU for the roll plate method and ≥100 CFU/catheter tip for sonication.
A total of 89 catheters were removed because of clinical suspicion of CRBSI and/or exit site infection with concomitant bacteremia. The mean dwell time for this subgroup was 56 days (range, 4 to 447 days). CRBSI in agreement with the aforementioned definitions was eventually diagnosed in 40 of these 89 catheter episodes (36 patients). Of these episodes, seven were in agreement with the definition of an exit site CRBSI, and in another six episodes, an endoluminal CRBSI was diagnosed. For the remaining 27 CRBSI, the distinction could not be made, which means that in these cases the hub cultures were negative and no signs of exit site infection were present but a DTTP of ≥2 h was recorded, or the result of a positive peripheral blood culture was concordant with the catheter tip culture. In 35 of the 40 episodes of CRBSI, antibiotic therapy with activity against the isolated microorganism had been administered in an attempt to salvage the catheter before the catheter tip was eventually cultured.
For this subgroup of 89 catheters with clinical suspicion of catheter-related infection, the diagnostic yields and predictive values were calculated for both techniques separately and in combination (Table 1). The sensitivity was disappointingly low for both catheter tip culture methods. In contrast, for both techniques the specificity and positive predictive values were better.
TABLE 1.
Diagnostic parameters for both tip culture techniques applied to catheters removed for suspected CRBSI or exit site infection with bacteremia (n = 89)a
| Method | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| Sonication | 45 (30-61) | 84 (70-92) | 69 (48-85) | 65 (52-76) |
| Roll plate | 35 (21-52) | 90 (77-96) | 74 (49-90) | 63 (50-74) |
| Combined data | 48 (32-64) | 84 (70-92) | 70 (50-86) | 63 (50-74) |
Data are percentages(95% confidence intervals). Cutoffs for detection of tip colonization were ≥15 CFU for the roll plate method and ≥100 CFU/catheter FSsegment for sonication.
Finally, we stratified these 89 catheters according to dwell time, creating groups with the median value (28 days) as a cutoff, to compare results for long-term and very-long-term catheters. Sensitivity, specificity, and predictive values for these subgroups were not different from those for the complete set of 89 catheters (data not shown).
DISCUSSION
In this study, we demonstrated that the use of the quantitative sonication technique to detect catheter tip colonization in patients with long-term tunnelled CVCs had no surplus value compared with the semiquantitative roll plate method. In addition, the diagnostic value of a catheter tip culture for patients with a tunnelled catheter under clinical suspicion of having a CRBSI seems limited, regardless of the method used. For both techniques, the diagnostic yield was lower if a culture technique was performed after the other one than if it was performed first. This is partly in accordance with the observation made by Sherertz and colleagues (23), who observed the same for sonication.
The fact that 35 of the 40 patients with CRBSI received antimicrobial treatment prior to catheter tip culture is a likely explanation for the observed low sensitivity of tip culture. Antibiotic therapy in an attempt to salvage the catheter will almost inevitably be given to patients with suspected CRBSI from a tunneled catheter. It is conceivable that this will lower the bacterial culture yield, both from the outer surface and from the endoluminal surface. Because these antimicrobial agents are administered through the CVC, endoluminal microorganisms are exposed to much higher antibiotic concentrations than are exoluminal bacteria. Antimicrobial pretreatment may therefore influence the sensitivity of the sonication method in particular. The negative impact of antimicrobial pretreatment on the diagnostic yield of catheter tip culture was recently demonstrated for short-term catheters (26).
Other explanations for why the sonication method did not perform better than the roll plate method are possible. In this study, all catheters were equipped with a disinfectable needle-free closed connector system. If used properly, this may decrease the risk of endoluminal CRBSI (29). Finally, the sonication technique may not be able to remove microorganisms from the endoluminal biofilm sufficiently.
The practice of pretreatment with antibiotics does raise the question of whether using lower cutoff values for colonization detection might be beneficial. In a separate analysis applied to patients with clinical suspicion of CRBSI, data obtained with Maki et al.'s method were recalculated using modified cutoffs for colonization, considering any growth of microorganisms on catheter tips concordant with the yield of blood cultures as a positive result. This did indeed improve the sensitivity of Maki et al.'s method from 35 to 58%, at only a limited cost of specificity (86 instead of 90%). Future studies should be performed to investigate this observation more specifically before stating that lower cutoffs may be preferred for patients receiving antimicrobial therapy before tip culture. For sonication, we did not evaluate the sensitivity of cutoffs below 100 CFU/catheter because this would have implied the inoculation of the total of 5 ml of sonication fluid on at least 10 agar plates, which we decided not to do because it would be very labor-intensive and therefore unacceptable in routine patient care.
To our knowledge, this is the first prospective, randomized study in which the conventional roll plate method was compared with sonication for patients with long-term tunnelled CVCs. In earlier studies, both techniques were compared with other, nonhomogenous patient populations with short-term devices (3, 11, 13, 18, 23, 28). In a recent study of 1,000 short-term CVCs, Bouza et al. demonstrated sonication (1 min at 55,000 Hz and 125 W) to be less sensitive than the roll plate method. For the roll plate method, a breakpoint of ≥15 CFU was used in this study, and for sonication, cutoffs of both ≥100 and ≥1,000 CFU/catheter segment were studied, of which ≥100 CFU/catheter segment demonstrated superiority for detection of tip colonization. However, for the subgroup of long-term catheters (defined as >6 days), the sensitivities of both methods were comparable (3). Unequivocally, the hypothesis that sonication could have additional diagnostic value due to its ability to detect endoluminal microorganisms is attractive. It has been suggested that the endoluminal route of catheter infection becomes dominant over the exit site as the source of infection in patients with long-term devices (15, 16, 19, 22). This may explain why sonication gave slightly better, although not significant, results than those by the roll plate method for the “long-term” subgroup of the study by Bouza et al. Taking into account that in this study long-term use was defined as >6 days suggests that these results could be even more pronounced if truly long-term catheters are studied, as in this study. However, we were unable to confirm this hypothesis. In a sample of 313 CVCs and arterial catheters from a mixed patient population, Raad and colleagues found fairly better diagnostic parameters for sonication (at 55,000 Hz and 125 W) than for the roll plate method. For the roll plate method, cutoff levels of ≥15 CFU to ≥1,000 CFU were studied, and for sonication, breakpoints of ≥102 to ≥104 CFU were evaluated. However, considering the results obtained by using the same breakpoints as those in our study, levels of sensitivity, specificity, and positive and negative predictive values for CRBSI were reported to be 78%, 88%, 35%, and 98%, respectively, by using the roll plate method, compared with 93%, 94%, 72%, and 99%, respectively, for sonication. No details are given on statistical significance, and for the given values only one of both procedures was performed on a single catheter tip (18). Also, Sherertz et al. reported better sensitivity with sonication than with the roll plate method (53% versus 33%; P < 0.05), using cutoffs of ≥15 CFU for the roll plate method and ≥100 CFU/catheter segment for sonication, for intensive care unit patients (23). Other researchers did not find differences in diagnostic performance between both techniques (11, 13, 18, 28).
According to current guidelines, routinely culturing the catheter tip is not recommended to avoid overtreatment of clinically insignificant tip colonization in patients without suspicion of CRBSI. Therefore, we determined sensitivity, specificity, and predictive values for both techniques for the subset of catheter episodes in which there was clinical suspicion of CRBSI. Establishing the diagnosis of CRBSI in these patients is preferably done by means of noninvasive diagnostic tests while the catheter is left in place. However, a reliable diagnostic test that can confirm or reject CRBSI in cases when the catheter is eventually removed would be helpful. The positive predictive value observed in this study could help to establish the diagnosis of CRBSI, but the low sensitivity does not allow the use of tip culture to reject the diagnosis of CRBSI.
In conclusion, for patients with long-term tunnelled CVCs, the diagnostic yields of the roll plate and sonication methods were comparable, although the sensitivities of both methods were low. This might be due to attempts to salvage the catheter by administering antibiotics in cases of suspected CRBSI to most of these patients in the days before catheter removal and tip culture. With this respect, our observation that lowering conventional tip colonization cutoffs can improve diagnostic accuracy could be valuable.
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Bjornson, H. S., R. Colley, R. H. Bower, V. P. Duty, J. T. Schwartz-Fulton, and J. E. Fischer. 1982. Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery 92720-727. [PubMed] [Google Scholar]
- 2.Blot, F., G. Nitenberg, E. Chachaty, B. Raynard, N. Germann, S. Antoun, A. Laplanche, C. Brun-Buisson, and C. Tancrede. 1999. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet 3541071-1077. [DOI] [PubMed] [Google Scholar]
- 3.Bouza, E., N. Alvarado, L. Alcala, M. Sanchez-Conde, M. J. Perez, P. Munoz, P. Martin-Rabadan, and M. Rodriguez-Creixems. 2005. A prospective, randomized, and comparative study of 3 different methods for the diagnosis of intravascular catheter colonization. Clin. Infect. Dis. 401096-1100. [DOI] [PubMed] [Google Scholar]
- 4.Brun-Buisson, C., F. Abrouk, P. Legrand, Y. Huet, S. Larabi, and M. Rapin. 1987. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch. Intern. Med. 147873-877. [PubMed] [Google Scholar]
- 5.Cleri, D. J., M. L. Corrado, and S. J. Seligman. 1980. Quantitative culture of intravenous catheters and other intravascular inserts. J. Infect. Dis. 141781-786. [DOI] [PubMed] [Google Scholar]
- 6.Constantinou, D., G. G. Geesey, and M. H. Wilcox. 1981. Abstr. Annu. Meet. Am. Soc. Microbiol., abstr. L13, p. 80. American Society for Microbiology, Washington, DC.
- 7.Edgeworth, J. D., D. F. Treacher, and S. J. Eykyn. 1999. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit. Care Med. 271421-1428. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis, W. R., J. R. Edwards, D. H. Culver, J. M. Hughes, T. Horan, T. G. Emori, S. Banerjee, J. Tolson, T. Henderson, R. P. Gaynes, et al. 1991. Nosocomial infection rates in adult and pediatric intensive care units in the United States. Am. J. Med. 91185S-191S. [DOI] [PubMed] [Google Scholar]
- 9.Kite, P., B. M. Dobbins, M. H. Wilcox, W. N. Fawley, A. J. Kindon, D. Thomas, M. J. Tighe, and M. J. McMahon. 1997. Evaluation of a novel endoluminal brush method for in situ diagnosis of catheter related sepsis. J. Clin. Pathol. 50278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluger, D. M., and D. G. Maki. 1999. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 514. American Society for Microbiology, Washington, DC.
- 11.Kristinsson, K. G., I. A. Burnett, and R. C. Spencer. 1989. Evaluation of three methods for culturing long intravascular catheters. J. Hosp. Infect. 14183-191. [DOI] [PubMed] [Google Scholar]
- 12.Linares, J., A. Sitges-Serra, J. Garau, J. L. Perez, and R. Martin. 1985. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J. Clin. Microbiol. 21357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki, D. G., L. A. Mermel, M. Martin, and D. Berry. 1996. Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 62-J. American Society for Microbiology, Washington, DC.
- 14.Maki, D. G., C. E. Weise, and H. W. Sarafin. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med. 2961305-1309. [DOI] [PubMed] [Google Scholar]
- 15.Mayhall, C. G. 1992. Diagnosis and management of infections of implantable devices used for prolonged venous access. Curr. Clin. Top. Infect. Dis. 1283-110. [PubMed] [Google Scholar]
- 16.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 321249-1272. [DOI] [PubMed] [Google Scholar]
- 17.Pittet, D., D. Tarara, and R. P. Wenzel. 1994. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 2711598-1601. [DOI] [PubMed] [Google Scholar]
- 18.Raad, I. I., M. F. Sabbagh, K. H. Rand, and R. J. Sherertz. 1992. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn. Microbiol. Infect. Dis. 1513-20. [DOI] [PubMed] [Google Scholar]
- 19.Raad, I. I., W. Costerton, U. Sabharwal, M. Sacilowski, E. Anaissie, and G. P. Bodey. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168400-407. [DOI] [PubMed] [Google Scholar]
- 20.Raad, I., H. A. Hanna, B. Alakech, I. Chatzinikolaou, M. M. Johnson, and J. Tarrand. 2004. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann. Intern. Med. 14018-25. [DOI] [PubMed] [Google Scholar]
- 21.Rijnders, B. J., E. Van Wijngaerden, and W. E. Peetermans. 2002. Catheter-tip colonization as a surrogate end point in clinical studies on catheter-related bloodstream infection: how strong is the evidence? Clin. Infect. Dis. 351053-1058. [DOI] [PubMed] [Google Scholar]
- 22.Safdar, N., J. P. Fine, and D. G. Maki. 2005. Meta-analysis: methods for diagnosing intravascular device-related bloodstream infection. Ann. Intern. Med. 142451-466. [DOI] [PubMed] [Google Scholar]
- 23.Sherertz, R. J., S. O. Heard, and I. I. Raad. 1997. Diagnosis of triple-lumen catheter infection: comparison of roll plate, sonication, and flushing methodologies. J. Clin. Microbiol. 35641-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherertz, R. J., I. I. Raad, A. Belani, L. C. Koo, K. H. Rand, D. L. Pickett, S. A. Straub, and L. L. Fauerbach. 1990. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J. Clin. Microbiol. 2876-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegman-Igra, Y., A. M. Anglim, D. E. Shapiro, K. A. Adal, B. A. Strain, and B. M. Farr. 1997. Diagnosis of vascular catheter-related bloodstream infection: a meta-analysis. J. Clin. Microbiol. 35928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souweine, B., A. E. Heng, C. Aumeran, F. Thiolliere, N. Gazuy, P. Deteix, and O. Traore. 2008. Do antibiotics administered at the time of central venous catheter removal interfere with the evaluation of colonization? Intensive Care Med. 34286-291. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel, R. P., and M. B. Edmond. 2001. The impact of hospital-acquired bloodstream infections. Emerg. Infect. Dis. 7174-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widmer, A. F. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-2036. American Society for Microbiology, Washington, DC.
- 29.Yebenes, J. C., M. Delgado, G. Sauca, M. Serra-Prat, M. Solsona, J. Almirall, J. A. Capdevila, and X. Balanzo. 2008. Efficacy of three different valve systems of needle-free closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit. Care Med. 362558-2561. [DOI] [PubMed] [Google Scholar]