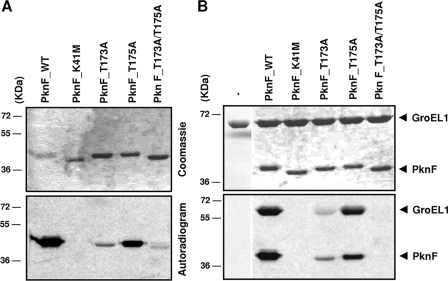
FIG. 2.
In vitro phosphorylation of M. tuberculosis GroEL1 by PknF and the different PknF mutants. (A) Autokinase activity of the different derivatives of PknF. All proteins [PknF(WT), PknF(K41M), PknF(T173A), PknF(T175A), and PknF(T173A/T175A)] were overproduced in E. coli, purified as His-tagged fusions, and incubated with [γ-33P]ATP. Proteins were separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). (B) In vitro phosphorylation of GroEL1 by PknF and the different PknF mutants in the presence of [γ-33P]ATP. Proteins were separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). The positions of molecular mass markers (in kilodaltons) are shown to the left of the gels.