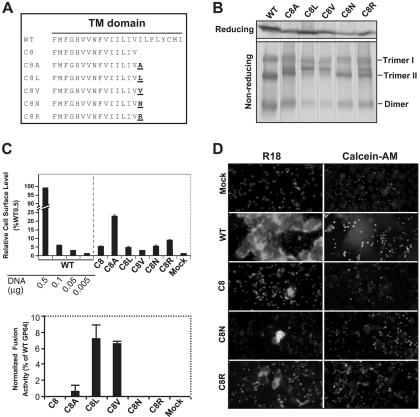
FIG. 4.
Analysis of the fusion requirements for GP64 construct C8. A single hydrophobic (A, L, or V) or hydrophilic (R or N) amino acid was added to the C terminus of the TM domain of the fusion-defective GP64 construct C8, and each resulting construct was then analyzed for restoration of membrane fusion activity. (A) Construction of GP64 constructs. The TM domain sequences of the fusion-defective C8 construct and the other constructs are shown below that of the WT GP64; amino acids added to the C terminus of the TM domain of C8 are indicated in underlined bold, and the construct name is indicated on the left. (B) Expression and trimerization of modified GP64 proteins. Sf9 cells were transfected with plasmids expressing either WT GP64 or modified GP64 construct C8A, C8L, C8V, C8N, or C8R, and expression and trimerization of GP64 were examined by Western blot analysis of cell extracts on reducing (top) and nonreducing SDS-PAGE gels (bottom). The positions of oligomeric forms (Trimer I and II and Dimer) are indicated on the right. (C) Analysis of relative cell surface levels and fusion activities of GP64 constructs. Relative cell surface levels of GP64 constructs were measured by cELISA (as described in the Fig. 2 legend). Each mutant GP64 construct was expressed by transfecting 0.5 μg plasmid DNA encoding the modified GP64 construct. Relative fusion activity was evaluated by using efficiency of syncytium formation, as described earlier (Fig. 2 legend). For each sample, five fields were examined. The means and standard deviations of the results of triplicate transfections are shown. (D) Analysis of hemifusion and fusion pore formation by GP64 protein constructs. Fluorescence micrographs show either membrane dye transfer (R18) or cytosolic dye transfer (Calcein-AM). Each mutant GP64 construct (or mock control) used for transfection is indicated at the left, and assays were performed as described in Materials and Methods and the Fig. 3 legend.