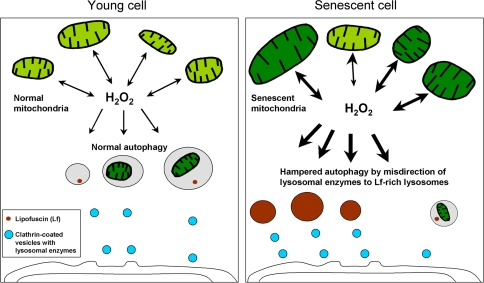
Fig. 8.
The mitochondrial–lysosomal axis theory of ageing. This hypothesis tries to explain the relationship between lipofuscin accumulation, decreased autophagy, increased ROS production, and mitochondrial damage in senescent long-lived postmitotic cells. Mitochondria are damaged by self-produced hydrogen peroxide, at least if redox-active iron is present. In young cells, damaged mitochondria are rapidly autophagocytosed and degraded because enough lysosomal enzymes are distributed throughout the lysosomal compartment. Small amounts of hydrogen peroxide that normally diffuse into lysosomes lead to cross-linking of intralysosomal material and consequent gradual accumulation of lipofuscin in long-lived postmitotic cells that can neither be degraded nor exocytosed. By purely random reasons, senescent cells have an increased amount of lysosomal enzymes directed towards the plentiful lipofuscin-rich lysosomes. These enzymes are lost for effective autophagic degradation while the lipofusicin remains non-degradable. The consequence for the senescent postmitotic cell is further decreased autophagy, a gradual accumulation of damaged mitochondria, increased hydrogen peroxide production, and apoptotic cell death