Abstract
Vaginal distension (VD) in outbred rats has been shown to decrease urethral resistance, as well as increase the expression of the stem cell-homing chemokine, monocyte chemotactic factor 3 (MCP-3), but not stromal derived factor 1 (SDF-1). The aim of this study was to determine if similar responses are induced by VD in an inbred rat strain. Forty female Lewis rats underwent VD or sham VD followed by leak point pressure (LPP) testing 4 or 10 days later. Ten additional rats served as controls. The urethra and vagina were then dissected for histology. To examine chemokine expression, 8 additional rats underwent VD with organs harvested immediately or 1 day after the procedure for RT-PCR of MCP-3 and SDF-1. Four age-matched rats served as controls. Four days after VD, LPP was significantly lower in VD rats (14.3±1.6 cmH2O) than controls (18.7±1.3 cmH2O). Ten days after VD, LPP in both VD (19.7±2.6 cmH2O) and sham (18.4±1.3 cmH2O) groups was not significantly different from controls. Urethral histology demonstrated marked disruption and atrophy of smooth and striated muscle in VD rats compared to shams and controls. RT-PCR yielded a 25-fold significant increase in expression of urethral MCP-3 immediately following VD. SDF-1 was significantly decreased in the urethra and vagina immediately after VD and in the bladder 24 hours after VD. In conclusion, VD in Lewis rats produces functional, histological and molecular results similar to that of outbred rats. This model could be utilized in future studies investigating cellular transplant methods of improving urethral function.
Keywords: Lewis rat, vaginal distension, stress urinary incontinence, leak point pressure, chemokines
Introduction
Urinary incontinence (UI) is a common disorder reported by 38-41% of adult women (1). Stress urinary incontinence (SUI) is the most prevalent type, accounting for 50% of UI (2). Vaginal childbirth can injure the nerve, muscle, and collagenous tissues responsible for maintaining continence (3). The prevalence of SUI increases from 4.7% in nulliparous women to 12.2% in women who have undergone vaginal delivery (4), suggesting a significant effect of parity. However, the symptoms of SUI often do not appear until years after the vaginal delivery (4;5).
To better understand the injury process of vaginal delivery, rat models of simulated maternal childbirth injury have been developed, utilizing vaginal distension (VD) to mimic the trauma associated with 2nd stage of labor in women (6;7). Whether subjected to VD after delivery or as virgins, rats develop decreased urethral resistance to leakage after VD, indicative of SUI (8-11). In addition, they demonstrate significant disruption of the external urethral sphincter (EUS), evidence of hypoxia, and sensitivity to duration of distension (6;10;12).
Stem cell therapies are among the newest strategies under investigation for the treatment and prevention of SUI. Direct localized injection (13-15) and targeted stem cell homing (16-18) are potential modalities that may one day be used to treat the symptoms of SUI. Homing of hematopoetic and mesenchymal stem cells (HSC & MSC) occurs in response to local upregulation of stem cell homing cytokines, such as monocyte chemotactic factor 3 (MCP-3) and stromal derived factor 1 (SDF-1) in response to injury, such as myocardial infarction or VD (16-19). The resultant cytokine gradient targets HSC and MSC to the area that has been injured, where they engraft, produce growth factors, facilitate healing from injury, and improve functional recovery (19). Thus, facilitation or duplication of endogenous stem cell homing may represent a method of stem cell treatment for SUI.
Thus far, investigations of simulated childbirth have used Sprague-Dawley (SD) rats, an outbred, all-purpose strain utilized in virtually all areas of biomedical research (7;9;10). In contrast, Lewis rats and other inbred rodent strains are predominantly utilized in immunologic, transplant, and stem cell research, owing to their clonal and syngeneic properties (20). The study of stem cell therapy for the treatment of SUI would therefore be optimized in an inbred strain; however the validity of the VD model in an inbred rat strain has not yet been demonstrated.
The aim of this study is to determine the effects of VD on urethral function and anatomy as well as on expression of the stem cell homing cytokines, MCP-3 and SDF-1, in the Lewis rat. We hypothesized that VD will result in reduced leak point pressure, disruption of the EUS, and upregulation of MCP-3 and SDF-1 in the urethra and vagina in this inbred strain.
Methods and Materials
Forty age-matched nulliparous female Lewis rats (weight: 190-220g; age: 10-12 weeks old) were randomized to undergo VD (n=20) or sham distension (n=20). Each group was further randomized to undergo leak point pressure (LPP) testing at 4 (n=10) or 10 (n=10) days after the procedure. Another group of 10 animals served as age-matched controls. A suprapubic bladder catheter was surgically implanted in each animal 2 days prior to LPP. Following LPP determination, animals were euthanized and the urethra, bladder and vagina were removed en bloc and immersion-fixed in 10% formalin for histological analysis.
An additional eight female Lewis rats were utilized for determination of MCP-3 and SDF-1 cytokine expression by RT-PCR either immediately or 24 hours after VD. These time points were selected since these cytokines are rapidly upregulated after injury (16;19) as we have previously observed in SD rats (17;18). Four unmanipulated age- and parity-matched nulliparous female Lewis rats served as controls. The bladder, urethra, vagina, and anterior rectum of each animal were dissected and flash-frozen at -80°C for reverse transcriptase polymerase chain reaction (RT-PCR) to assess upregulation of SDF-1 and MCP-3. Details of procedures are below.
Vaginal Distension (VD)
Rats were anesthetized with a mixture of intraperitoneal (i.p.) ketamine (100 mg/kg) and xylazine (10 mg/kg). The vagina was first accommodated to a larger capacity by inserting and removing increasing sizes of urethral dilators (24Fr. to 32Fr.). A trimmed 10Fr. Foley catheter was inserted intravaginally and the balloon inflated with water to 3 ml as previously described (9;10). The catheter was secured with a single suture and left in place for 4 hours. Animals in the sham distension groups underwent vaginal accommodation with the urethral dilators as well as insertion of the catheter for 4 hrs, without inflation of the balloon.
Suprapubic Bladder Catheter Implantation (SPT)
Animals were anesthetized with ketamine and xylazine as above and an abdominal midline incision was made 0.5 cm cephalad to the urethral meatus for suprapubic bladder catheterization as we have done previously (10). After localization of the bladder, a circular purse-string suture (5-0 chromic) was placed in the anterior vesical wall. A puncture incision was then made in the bladder wall, in the center of the purse-string stitch, and the suprapubic catheter (PE-50 tubing modified with a flared tip and proximal drainage hole) was inserted through this incision and secured with the suture. The catheter was subcutaneously tunneled to exit the skin at the level of the neck and plugged with a stainless steel stopper. The exit site around the catheter and the lower abdominal incision were closed with 3-0 vicryl sutures.
Leak Point Pressure (LPP) Determination
Rats were anesthetized with urethane (1.2 g/kg i.p.) and placed supine. The bladder was emptied, and room temperature saline was infused (5 ml/hr) via the previously-implanted suprapubic catheter. Bladder pressure was measured via the bladder catheter (P300, Grass Instruments, West Warwich, RI). Pressure was amplified (model P122, Grass Instruments), recorded on a chart recorder, and digitized (10 samples/sec) for computer data collection. After instillation of approximately 0.5 ml saline, a gentle slow manual pressure increase was applied to the rat's abdomen by a trained investigator blinded to the specific injury until urethral leakage occurred, simulating a mild Crede maneuver as previously described (9;10). We have previously shown that consistency is achievable if the investigator is trained and the rate of pressure increase is slow (21).
If spontaneous voiding occurred, the bladder was refilled and LPP was remeasured. External pressure at the moment of leakage in the absence of a bladder contraction, calculated as the baseline pressure subtracted from peak pressure, was taken as the LPP. The test was repeated twice in each rat, and a mean value of LPP for each rat was calculated and used to produce group mean ± standard error values.
Histological Tissue Analysis
The bladder, urethra, and vagina, preserved en bloc in 10% formalin, were micro-dissected as follows. The bladder was opened to locate the ureteral orifices inside the bladder lumen. Measurements were taken to locate a spot on the urethra 5mm distal to the ureteral orifice, the level of maximum striated muscle area (22). A segment of the urethra attached to the anterior portion of the vagina with proximal end 5mm distal to the ureteral orifice, was removed and underwent tissue processing and paraffin embedding. The tissue was sectioned (5 μm) at the proximal end of the specimen, approximately 5 mm distal to the ureteral orifice as previously described (22). Uniformity of sectioning was ensured by standardizing the level of the urethra and by processing all specimen as a batch. Slides were stained with Masson's Trichrome and qualitatively compared in a blinded fashion. Atrophy was defined qualitatively as a paucity of muscle fibers. Muscle disruption was defined qualitatively as a dramatic and consistent shortening and disconnection of striated muscle fibers compared to controls.
RT-PCR for MCP-3 and SDF-1
Total RNA was isolated from frozen tissues using RNeasy Mini RNA isolation kit (Qiagen, Valencia, CA) as we have previously described (17;18). RNA concentration was determined using a Ribogreen assay (Molecular Probes, Eugene, OR). Contaminating DNA was removed by using DNA-free, DNase Treatment and Removal Reagent #1906 (Ambion Inc., Austin, TX) and reverse transcription reaction was performed in 20 μl of reaction volume with 400 ng of total RNA, 2.5 μM oligo dT reverse transcription primer, and 1 unit of reverse transcriptase at 48°C for 45 minutes. Eighty ng of input cDNA was used for TaqMan® quantitative real-time RT-PCR (SYBR Green PCR Master Mix: PN 4309155, Applied Biosystems, Foster City, CA). Amplification was targeted to MCP-3 or SDF-1 mRNA using forward and reverse primer pairs (Applied Biosystems, Foster City, CA). The same amount of cDNA was used for SYBR Green TaqMan® PCR of GAPDH (Applied Biosystems, Foster City, CA) as the endogenous control. Two-step TaqMan® PCR was performed at a 25 μl reaction volume using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Cycle conditions were: 50°C hold for 2 minutes, 95°C hold for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Critical threshold values for each sample were determined and data were analyzed according to the manufacturer's protocol. RT-PCR results were normalized to GAPDH results for statistical comparisons between groups.
Statistical analysis of Quantitative Results
Quantitative data is presented as mean ± standard error of the mean of each experimental group. Statistical comparisons of LPP values were made using a t-test with Bonferroni correction for multiple comparisons. P < 0.05 was taken to indicate a significant difference between groups. The method of mixed models was used to test for significant differences between groups in normalized RT-PCR data. Pairwise comparisons were performed using a Tukey-Kramer adjustment for multiple means comparisons. A pairwise difference with P < .05 was considered to be statistically significant. RT-PCR data is presented normalized to mean of the control group for each organ.
Results
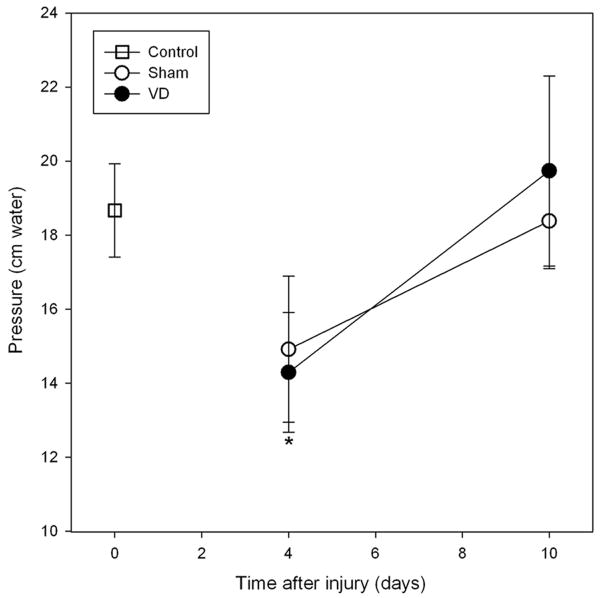
All 62 rats utilized in this study survived and were included in the final analysis. Lewis rats demonstrated LPP results similar to those observed previously in SD rats (9;10). No modifications to the experimental method of VD or LPP were required for Lewis rats. Four days after VD, LPP was significantly lower in VD rats (14.3±1.6 cm H2O) compared to controls (18.7±1.3 cm H2O; P< 0.05) but not to sham distended animals (14.9±2.0 cm H2O; p=0.8; Figure 1). Ten days after VD, LPP values in both VD (19.7±2.6 cm H2O) and sham VD (18.4±1.3 cm H2O) animals were not significantly different from controls (p=0.8).
Figure 1.
Leak point pressure (LPP) in control rats (open squares) and 4 or 10 days after vaginal distension (VD; filled circles) or sham distension (Sham; open circles) in Lewis rats. Each symbol represents the mean +/- standard error of data from 7-10 rats. * indicates a significant decrease in LPP 4 days after VD compared to the control group.
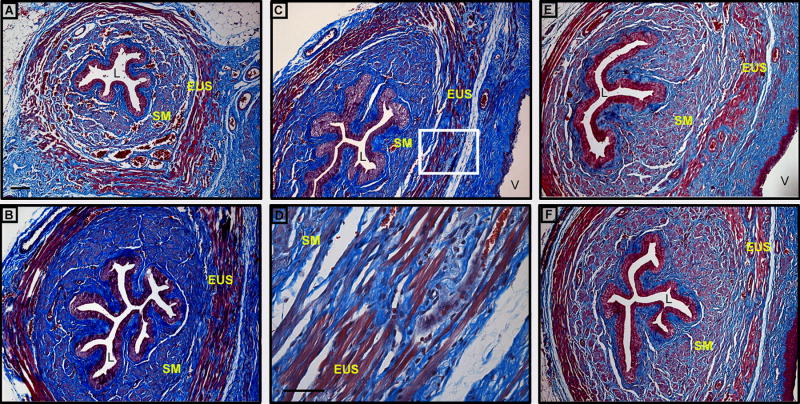
Four and 10 days after VD, urethral histology consistently demonstrated marked disruption and atrophy of smooth and striated muscle layers with mild thinning of muscle fibers in vaginally distended rats compared to sham distended rats and controls (Figure 2). These animals also had a noticeable paucity of striated muscle fibers in the vaginal side of the urethra and focal infiltrates in the EUS.
Figure 2.
Light micrographs of transverse sections of urethra in Lewis rats: A. control, B. 4 days after sham VD, C. and D. 4 days after VD, E. 10 days after VD, and F. 10 days after sham VD; Region of higher magnification (40×) in D is indicated by box in C. Masson's trichrome, Magnification = 10× (A, B, C, E, F); Bar = 10 μm (A and D); L = lumen, EUS = external urethral sphincter, SM = smooth muscle, V = vagina.
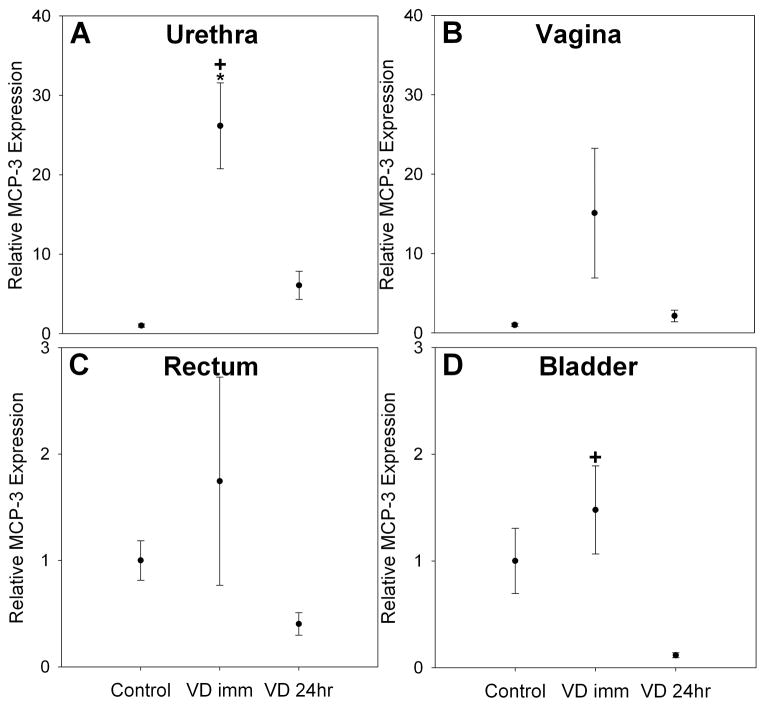
Semi-quantitative RT-PCR for MCP-3 demonstrated that MCP-3 mRNA was significantly upregulated in the urethra (25-fold increase, P< 0.009) immediately following VD compared to controls. MCP-3 mRNA decreased in the urethra 24 hours after VD and was significantly lower than immediately after VD but no longer significantly different from controls (Figure 3). MCP-3 expression was upregulated immediately after VD in the bladder, but not significantly so. It was significantly downregulated in the bladder 24 hours after VD compared to immediately after VD (Figure 3). Significant MCP-3 upregulation was not observed either in the vagina, rectum or bladder immediately after VD compared to controls, although MCP-3 over-expression in the vagina was observed immediately after VD (15-fold increase) compared to control and returned to control values 24 hours after VD.
Figure 3.
Monocyte chemotactic protein 3 (MCP-3) expression in the urethra (A), vagina (B), rectum (C) and bladder (D) of Lewis rats in control rats as well as immediately (VD imm), and 24 hours after VD (VD 24hr). Each bar represents the mean +/- standard error of the mean of data from 4 rats. * indicates a significant difference compared to comparable control; + indicates a significant difference compared to 24 hours after VD. Values on y-axis are relative expression after normalization to GAPDH results for each specimen and subsequently to the mean of the control group for each organ. Note that Y-axes are identical for urethra and vagina (A & B) as well as for rectum and bladder (C & D).
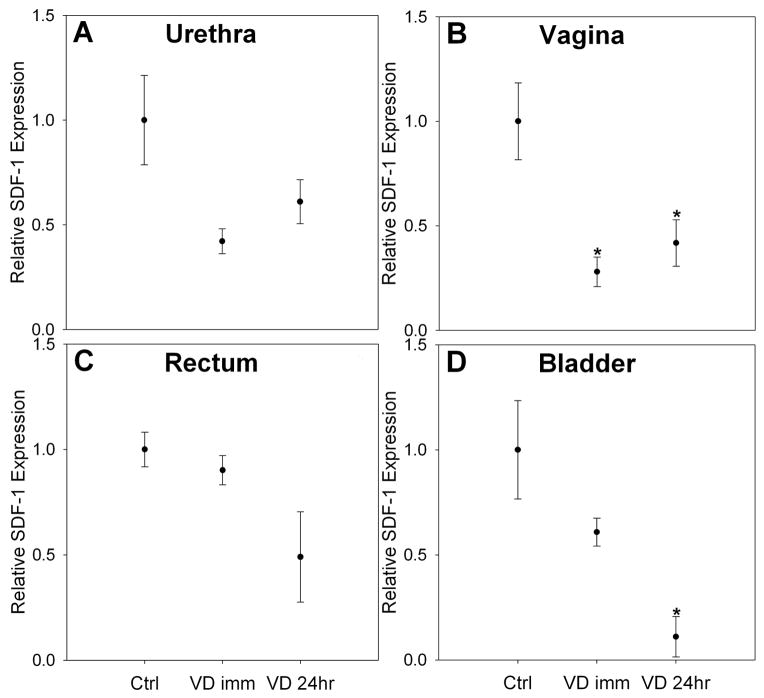
SDF-1 expression in the urethra and vagina was significantly downregulated immediately after VD compared to control (Figure 4). SDF-1 expression in the urethra returned to control levels 24 hours after VD, but remained significant downregulated in the vagina 24 hours after VD. There was no significant difference in SDF-1 expression in the rectum or bladder immediately following VD, but a significant down-regulation was seen in bladder tissues 24 hours after VD compared to the control group.
Figure 4.
Stromal derived factor 1 (SDF-1) expression in the urethra (A), vagina (B), rectum (C) and bladder (D) of Lewis rats in control, as well as immediately (VD imm), and 24 hours after VD (VD 24hr). Each bar represents the mean +/- standard error of the mean of data from 4 rats. * indicates a significant difference compared to comparable control. Values on y-axis are relative expression after normalization to GAPDH results for each specimen and subsequently to the mean of the control group for each organ.
Discussion
Rats are among the most widely-utilized animal models for the study of SUI and have shed light on possible etiologic factors and potential treatments for incontinence. A rat model simulating human childbirth injury was first introduced by Lin et al, in which artificial VD resulted in urethral leakage during subsequent sneeze testing (7). Cannon et al went on to demonstrate significantly lowered urethral resistance to leakage in rats that had previously undergone VD (6;9;10). These findings suggest a possible relationship between pelvic floor injuries incurred during vaginal delivery of children and the subsequent development of SUI. These studies have utilized the outbred SD rat, a cost-effective, widely used and versatile research model. Outbred stocks such as the SD rat are ideal as an all-purpose general research subject for experiments in which considerations of specific genotype may be of less importance (20).
Inbred rat strains, such as the Lewis rat, are characterized by genetic uniformity and the clonal nature of these strains renders them better-suited for use in transplantation and immunology research (23) since immunologic rejection becomes less of a concern. However, a greater susceptibility to stress, inflammatory disease states, induced autoimmune disorders, and certain pharmaceuticals has been documented in the Lewis rat (20).
The evolution of stem cell-mediated forms of incontinence therapy has recently gained attention (8;13). The immunologic implications of this research require immune-tolerant animal models, so that rejection does not complicate the results. Syngeneic rats, such as Lewis rats are ideal for this research and have typically been utilized in experiments involving cell or tissue transfer in stem cell research, transplantation, and tumor models (23;24).
Whether urological findings in the SD rat can be extrapolated across or applied to other rat strains has not been previously established. Despite apparent physical similarities, significant physiologic and anatomic differences have been observed among different rat strains (23-25). Given the different physiologic responses and outcomes that have been observed between inbred and outbred strains, it is important to validate the responses to VD in each strain prior to its utilization in studies investigating incontinence and potential treatments.
Lewis rats appeared to have lowered LPP values compared to SD rats (6;9;10). Like their SD counterparts, Lewis rats that underwent VD demonstrated a significant decrease in LPP when compared to controls, however not when compared to sham distended rats. This is in contrast to results in SD rats in which the LPP of sham rats is significantly higher than VD rats (6;9;10). This suggests increased susceptibility in Lewis rats to the sham distension procedure, although the mechanism of this response is not known.
The findings of muscle disruption in the urethra of Lewis rats following VD was similar in pattern to that reported previously in SD rats (6;9;10). These findings were absent in both sham and control animals, as was also observed previously in SD rats. Also similar to previous findings in SD rats (10), we observed that functional recovery occurs prior to histological recovery, suggesting a possible compensatory mechanism providing restored function prior to EUS recovery.
Several factors that may potentially contribute to VD-induced SUI have been previously postulated. Besides the direct tissue crush injury from the balloon, regional tissue hypoxia during VD with subsequent damage to pelvic floor muscles and the urethral sphincter, as well as pudendal nerve injury may also play contributing roles (7;9;12). Previous studies suggest that inbred rats are more resilient to ischemic injury compared to SD rats. In experimental stroke models, occlusion of the middle cerebral artery caused significantly larger ischemic lesion volumes in SD rats compared to Wistar, an inbred strain (26). A study of flap ischemia found that Lewis rats displayed a greater area of surviving flap tissue than SD rats for all ischemia times, suggesting improved capacity of Lewis rat tissue to withstand ischemic damage (23). These findings seemingly contradict the increased susceptibility of Lewis rats to sham VD, but might explain the relatively rapid recovery and return to control level 10 days after VD in Lewis rats. In comparison, 10 days after 4 hour VD in SD rats, LPP remains significantly decreased compared to controls (10). The mechanism of relatively rapid recovery in the Lewis rats is a topic for future investigations.
We have previously demonstrated significant upregulation of the stem-cell homing cytokine, MCP-3 in urethral and vaginal tissues following VD in SD rats (17;18). MCP-3 quantification in Lewis rats following VD demonstrated overexpression patterns in the urethra and vagina similar to that of SD rats, suggesting similar gene expression profiles between the two strains (17;18).
Also similar to SD rats, Lewis rats were found to have decreased levels of SDF-1 in urethral and vaginal tissues after VD (17;18). SDF-1 is a known homing molecule for hematopoietic stem cells and lymphocytes to various organ systems, including myocardium after myocardial infarction, brain after stroke and synovial tissues in the setting of rheumatoid arthritis (16;27). Recent studies have shown that SDF-1 levels are related to the degree of hypoxia, such that hypoxic gradients in tissues directly correlate with SDF-1 gene expression (28). SDF-1 was significantly under-expressed immediately after VD in all tissues in SD animals (17;18); however, it only showed a significant difference in the urethra and vagina, but not rectum and bladder in Lewis rats immediately after VD compared to control rats, suggesting that Lewis rats might have a different pattern of hypoxia after VD, consistent with experiments studying ischemia after stroke (27). Delayed expression of SDF-1 after VD is a possibility and will be investigated in future experiments.
While this study demonstrates similar but not identical responses to VD between Lewis and SD rats, it has several limitations. We used virgin rats since postpartum rats demonstrate increased variability (29). Although virgin rats do not have the hormonal milieu that is present after delivery, VD has been performed immediately postpartum with similar results (11). Therefore, while our model does not represent all aspects of childbirth, it nonetheless remains useful for investigations. Sham controls were not used in the comparison of cytokine upregulation. In addition, Western blots of MCP-3 and SDF-1 were not performed since the antibody for MCP-3 is not yet available. These are planned for future investigations.
Conclusions
We have validated the VD model of childbirth simulation in an inbred rat strain using functional, histological and molecular outcomes. Differences exist in the response to VD between Lewis and SD rats. Nevertheless, Lewis rats validate functional, histological, and molecular results previously observed in SD rats and can therefore be utilized in future studies investigating immunologic and stem cell interventions to facilitate recovery from childbirth injuries and resulting incontinence.
Acknowledgments
This material is based on work supported by NIH Grant RO1 HD038679, The Cleveland Clinic, and the office of Research and Development, Rehabilitation Research and Development Service of the Department of Veterans Affairs. The authors would like to thank R. Sam Butler for statistical advice.
References
- 1.Anger JAT, Saigal CS, Litwin MS. Urologic Diseases of America Project. The prevalence of urinary incontinence among community dwelling adult women: results from the National Health and Nutrition Examination Survey. Journal of Urology. 2006;175:423–4. doi: 10.1016/S0022-5347(05)00242-9. [DOI] [PubMed] [Google Scholar]
- 2.Contreras OO. Stress urinary incontinence in the gynecological practice. Gynecologic and Obstetric Investigation. 2004;86(Suppl 1):S6–S16. doi: 10.1016/j.ijgo.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HP. Levator function before and after childbirth. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2004;44:19–23. doi: 10.1111/j.1479-828X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 4.Rortveit G, Kjersti A, Hannestad Y, Hunskaar S. Urinary incontinence after vaginal delivery or Cesarean Section. New England Journal of Medicine. 2003;348:900–7. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 5.Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstetrics & Gynecology. 2006;108:873–8. doi: 10.1097/01.AOG.0000233172.96153.ad. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TW, Ferguson C, Wojcik EM, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU International. 2002;90(4):403–7. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin AS, Carrier S, Morgan DM, Lue TF. The effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52(1):143–51. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith C, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003 Nov;62(5):958–63. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 9.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. Journal of Urology. 2003 Sep;170(3):1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 10.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;292:R1738–R1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sievert KD, Bakircioglu ME, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: Functional and structural change. Journal of Urology. 2001;166(1):311–7. [PubMed] [Google Scholar]
- 12.Damaser MS, Whitbeck C, Chichester P, Levin RM. Reduced blood flow and hypoxia of urogenital organs of the female rat due to vaginal distension. Journal of Applied Physiology. 2005;98(5):1884–90. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 13.Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Frauscher F, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomized controlled trial. Lancet. 2007;369:2179–2186. doi: 10.1016/S0140-6736(07)61014-9. [DOI] [PubMed] [Google Scholar]
- 14.Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, et al. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008 Jan;53(1):169–75. doi: 10.1016/j.eururo.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Smaldone MC, Chancellor MB. Muscle derived stem cell therapy for stress urinary incontinence. World Journal of Urology. 2008 doi: 10.1007/s00345-008-0269-9. Epub ahead of print(PMID: 18470515) [DOI] [PubMed] [Google Scholar]
- 16.Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. International Journal of Cardiology. 2004 Jun;95(Suppl 1):S23–S25. doi: 10.1016/s0167-5273(04)90007-1. [DOI] [PubMed] [Google Scholar]
- 17.Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Overexpression of stem cell homing cytokines in rat pelvic organs following vaginal distension. Journal of Urology. 2007;177:1568–72. doi: 10.1016/j.juro.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 18.Wood HM, Kuang M, Woo LL, Hijaz A, Penn MS, Rackley RR, et al. Cytokine expression after vaginal distension of different durations in virgin Sprague-Dawley rats. Journal of Urology. 2008 doi: 10.1016/j.juro.2008.03.182. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte Chemotactic Protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 20.Festing MFW. Genetic variation in outbred rats and mice and its implications for toxicological screening. Journal of Experimental Animal Science. 1993;35:210–20. [PubMed] [Google Scholar]
- 21.Shoffstall AJ, Zaszczurynski P, Butler RS, Damaser MS. Development of a device to standardize leak point pressure experiments in rats. Neurourology & Urodynamics. 2008 doi: 10.1002/nau.20591. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim RJ, Kerns JM, Liu S, Nagel T, Zaszczurynski P, Lin DL, et al. Striated muscle and nerve fascicle distribution in the rat urethral sphincter. Anatomical Record. 2007;290:145–54. doi: 10.1002/ar.20420. [DOI] [PubMed] [Google Scholar]
- 23.Deune EG, Khouri RK. Rat strain differences in flap tolerance to ischemia. Microsurgery. 1995;16:765–7. doi: 10.1002/micr.1920161114. [DOI] [PubMed] [Google Scholar]
- 24.Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological Psychiatry. 2006;59:1208–18. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt C, Miranpuri GS, Dhodda VK, Isaacson J, Vemuganti R, Resnick DK. Changes in spinal cord injury-induced gene expression in rats are strain dependent. Spine. 2006;6:113–9. doi: 10.1016/j.spinee.2005.05.379. [DOI] [PubMed] [Google Scholar]
- 26.Walberer M, Stolz E, Muller C, Friedrich C, Rottger C, Blaes F, et al. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI) Lab Animal. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- 27.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. Journal of Neuropathology & Experimental Neurology. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Gurtner GC. Homing to hypoxia. HIF-1 as a mediator of progenitor cell recruitment to injured tissues. Trends in Cardiovascular Medicine. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, et al. Effects of pudendal nerve injury in the female rat. Neurourology and Urodynamics. 2000;19(1):53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]