Abstract
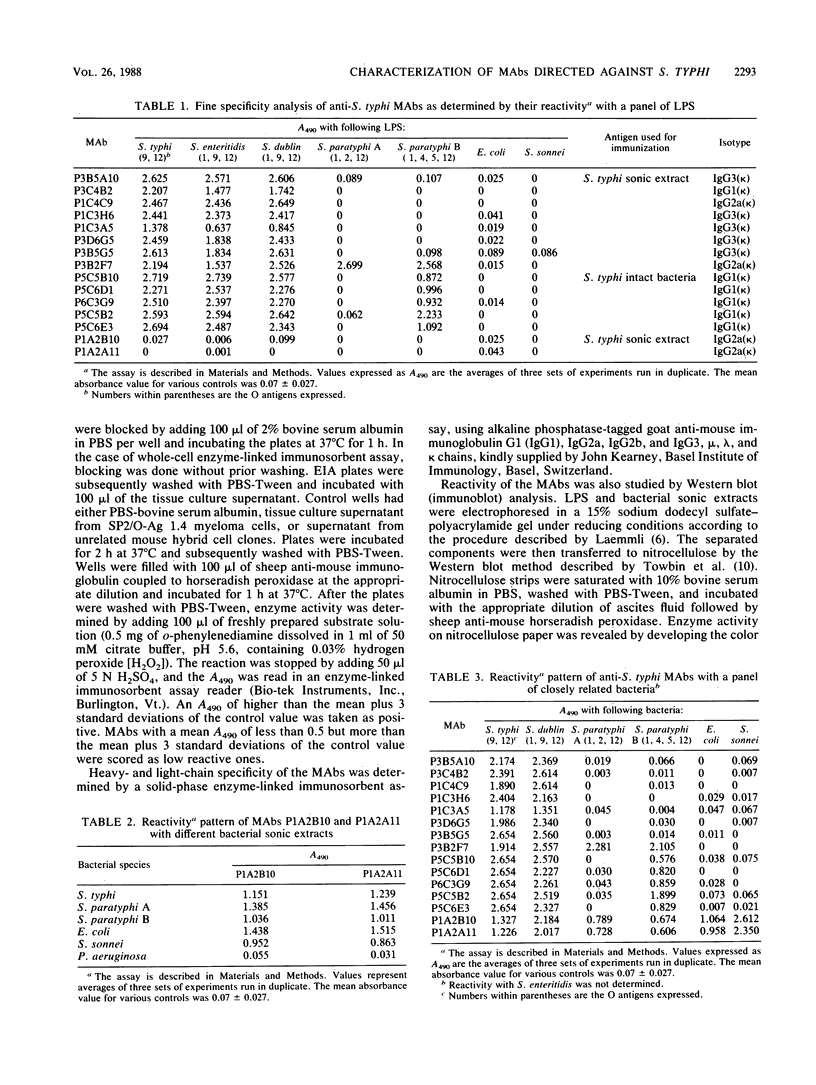
Fifteen monoclonal antibodies (MAbs) directed against Salmonella typhi were produced and characterized. The specificities of the antibodies were determined by their binding patterns in an enzyme immunoassay, with a panel of lipopolysaccharides isolated from different bacteria. Seven MAbs reacted with S. typhi, Salmonella enteritidis, and Salmonella dublin (all belonging to serogroup D). One MAb also reacted with Salmonella paratyphi A and S. paratyphi B. Five MAbs reacted with S. typhi, S. enteritidis, S. dublin, and S. paratyphi B. Two MAbs did not bind to any lipopolysaccharide but showed reactivity with bacterial sonic extracts isolated from S. typhi, S. paratyphi A, S. paratyphi B, Escherichia coli, and Shigella sonnei. These antibodies would be helpful in studying the complexity of antigenic determinants expressed by S. typhi and the nature of the antibody response during typhoid and paratyphoid fevers and also in the diagnosis of the disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Gupta S. K., Talwar G. P. Development of hybridomas secreting anti-human chorionic gonadotropin antibodies. Indian J Exp Biol. 1980 Dec;18(12):1361–1365. [PubMed] [Google Scholar]
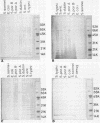
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sidberry H., Kaufman B., Wright D. C., Sadoff J. Immunoenzymatic analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985 Feb 11;76(2):299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Pollack M., Young L. S., Koles N., Gascon R., Pier G. B. Functionally active monoclonal antibody that recognizes an epitope on the O side chain of Pseudomonas aeruginosa immunotype-1 lipopolysaccharide. Infect Immun. 1986 Sep;53(3):656–662. doi: 10.1128/iai.53.3.656-662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]