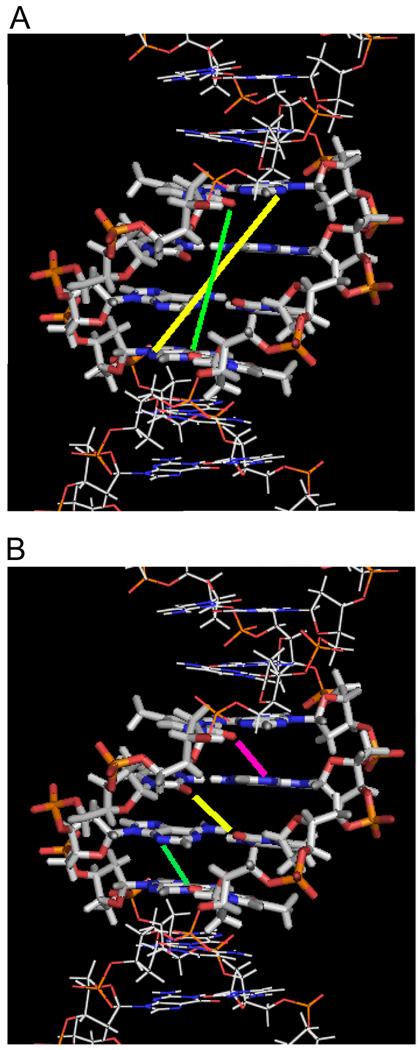
Figure 2.
Interstrand distances of H-bond acceptor groups at the AATT site in d(CGCGAATTCGCG)2. (A) Two sets of H-bond acceptors are within a four-base pair length. The N3 of A5 to N3 of A17 distance (yellow) is longer than the O2 of T8 to O2 of T20 distance (green), and it is a through-base distance so that a bound compound must have some curvature in order to form H-bonds with the two Ns. (B) The shortest inter-strand acceptor-acceptor distances (3.7 Å) within the minor groove of -AATT- are illustrated. The three lines indicate O2 of T8 to N3 of A18 (magenta), O2 of T7 to O2 of T19 (yellow), and N3 of A6 to O2 of T20 (green). Compound -NH- groups (from amidine, amide, benzimidazole, or indole) can form bifurcated H-bonds with these three pairs of H-bond acceptors.