Abstract
Organisms often make effort-related choices based upon assessments of motivational value and work requirements. Nucleus accumbens dopamine is a critical component of the brain circuitry regulating work output in reinforcement-seeking behavior. Rats with accumbens dopamine depletions reallocate their instrumental behavior away from food-reinforced tasks that have high response requirements, and instead they select a less-effortful type of food-seeking behavior. The ventral pallidum is a brain area that receives substantial GABAergic input from nucleus accumbens. It was hypothesized that stimulation of GABAA receptors in the ventral pallidum would result in behavioral effects that resemble those produced by interference with accumbens dopamine transmission. The present studies employed a concurrent choice lever pressing/chow intake procedure; with this task, interference with accumbens dopamine transmission shifts choice behavior such that lever pressing for food is decreased but chow intake is increased. In the present experiments, infusions of the GABAA agonist muscimol (5.0–10.0 ng) into the ventral pallidum decreased lever pressing for preferred food, but increased consumption of the less preferred chow. In contrast, ventral pallidal infusions of muscimol (10.0 ng) had no significant effect on preference for the palatable food in free-feeding choice tests. Furthermore, injections of muscimol into a control site dorsal to the ventral pallidum produced no significant effects on lever pressing and chow intake. These data indicate that stimulation of GABA receptors in ventral pallidum produces behavioral effects similar to those produced by accumbens dopamine depletions. Ventral pallidum appears to be a component of the brain circuitry regulating response allocation and effort-related choice behavior, and may act to convey information from nucleus accumbens to other parts of this circuitry. This research may have implications for understanding the brain mechanisms involved in energy-related psychiatric dysfunctions such as psychomotor retardation in depression, anergia, and apathy.
Keywords: anergia, motivation, nucleus accumbens dopamine, decision making, fatigue
Organisms are separated from significant stimuli by environmental constraints or obstacles (i.e. response or procurement “costs”), and therefore instrumental behaviors often are characterized by a high degree of vigor, persistence and work output (Salamone, 1992; Salamone and Correa, 2002; Salamone et al., 2007; Niv et al., 2007). The work requirements for obtaining reinforcing stimuli can differ substantially depending upon the instrumental task, and these requirements can vary along several distinct dimensions (e.g. numbers of responses, force or distance requirements; Collier and Jennings, 1969; Aberman and Salamone, 1999; Ishiwari et al., 2004; van den Bos et al., 2006). Furthermore, organisms often make effort-related choices based upon cost/benefit analyses; responses can be allocated in relation to several factors, including assessments of motivational value and work requirements (Salamone et al., 1991, 1997, 2003, 2005; Salamone and Correa, 2002; Denk et al., 2005; Rushworth et al., 2004; Ernst and Paulus, 2005; Phillips et al., 2007). Pathologies involving effort-related functions, such as psychomotor slowing, anergia, and apathy, are recognized as an important feature of various psychiatric syndromes, including depression and other disorders (Tylee et al., 1999; Stahl, 2002; Salamone et al., 2006). Considerable evidence indicates that dopamine (DA), particularly in nucleus accumbens, regulates effort-related processes (i.e. processes involved in overcoming work-related response costs; Salamone et al., 1991, 2003, 2005, 2007; Vezina et al., 2002; Zhang et al., 2003; Wakabayashi et al., 2004; Barbano and Cador, 2006, 2007; Cagniard et al., 2006; Denk et al., 2005; Phillips et al., 2007; Floresco et al., in press). Food-reinforced lever pressing tasks that have minimal work requirements are generally reported to be only minimally affected by accumbens DA depletions, while schedules that have high work requirements (e.g. large ratio requirements) are substantially impaired by accumbens DA depletions (Aberman and Salamone, 1999; Salamone et al., 2003; Ishiwari et al., 2004; Mingote et al., 2005). Moreover, interference with accumbens DA transmission can profoundly alter effort-related choice behavior. Research involving choice tasks has shown that rats given low doses of DA receptor antagonists, and rats with accumbens DA depletions, reallocate their behavior away from tasks that have high response requirements, and instead select less effortful types of food-seeking behavior (Salamone et al., 1991, 1994, 2002; Cousins et al., 1993, 1996; Koch et al., 2000; Nowend et al., 2001). In several studies, a concurrent lever pressing/chow feeding procedure has been used, in which rats have the choice to either work for a preferred food by lever pressing on a fixed ratio 5 (FR 5) schedule, or simply to approach and consume a less-preferred food (i.e. laboratory chow) that is concurrently available in the chamber (Salamone et al., 1991). Under baseline or control conditions, rats typically obtain most of their food by lever pressing, and interference with DA transmission by injections of low doses of DA antagonists or depletions of accumbens DA causes a dramatic shift in behavior characterized by reduced lever pressing for food accompanied by increased levels of chow intake (Salamone et al., 1991, 2002; Cousins et al., 1993; Koch et al., 2000; Nowend et al., 2001).
Although accumbens DA is a vital component of the brain circuitry regulating work output and effort-related choice behavior (Salamone and Correa, 2002; Salamone et al., 2003, 2005, 2006, 2007), it is recognized that other brain areas (e.g. anterior cingulate cortex, amygdala) and neurotransmitters (e.g. adenosine) must also be involved (Walton et al., 2002, 2003, 2006, 2007; Schweimer et al., 2005; Schweimer and Hauber, 2006; Floresco and Ghods-Sharifi, 2007; Salamone et al., 2006, 2007; Farrar et al., 2007). One of the brain areas receiving the greatest input from nucleus accumbens is the ventral pallidum (VP; Heimer et al., 1982; Zahm and Brog, 1992; Kretschmer, 2000). The projections from nucleus accumbens to VP are GABAergic (Groenewegen and Russchen, 1984; Zahm and Brog, 1992; Kalivas et al., 1993), and these neurons also colocalize substance P or enkephalin (Groenewegen and Russchen, 1984; Zahm and Brog, 1992; Kalivas et al., 1993). The VP is very rich in GABA, and approximately 85% of the axon terminals that form identifiable synapses in the VP are GABAergic (Chang et al., 1995). Neurons from VP project to mediodorsal thalamus and various brainstem motor areas (Zahm and Brog, 1992; Groenewegen et al., 1993). VP appears to function both as a relay station for information passing through from nucleus accumbens, and also as an integrator of information related to diverse limbic and striatal inputs (Koob and Swerdlow, 1988; Chrobak and Napier, 1993; Kretschmer, 2000). Previous studies have implicated VP GABA in prepulse inhibition of startle (Swerdlow et al., 1990; Kodsi and Swerdlow, 1995), and in functions related to drug reinforcement, including conditioned place preference and drug self-administration (Kretschmer, 2000; June et al., 2003; Koob, 2004; Caille and Parsons, 2004). Furthermore, several previous studies have illustrated the importance of ventral pallidal GABAA receptors in the modulation of locomotor activity (Jones and Mogenson, 1980; Mogenson and Nielsen, 1983; Austin and Kalivas, 1990; Hooks and Kalivas, 1995). However, less is known about the role of VP GABAA receptors in regulating food-reinforced instrumental behavior. VP neurons show increased firing rates in response to sucrose reward and pavlovian cues predicting reinforcement (Tindell et al., 2004), and VP lesions have been shown to reduce maximum rates of lever pressing reinforced by food (Johnson et al., 1996). Injections of 100–200 ng of bicuculline into the VP increased food intake, but did not affect hedonic responses to sucrose (Smith and Berridge, 2005).
The present studies were undertaken to investigate the role of VP GABAA receptors in modulating response allocation in food-reinforced behavior. Experiment 1 studied the effects of VP injections of the GABAA agonist muscimol on performance of the concurrent lever pressing/chow feeding task. In view of data indicating that stimulation of GABAA receptors and accumbens DA depletions can produce similar effects on locomotion and prepulse inhibition of startle, it was hypothesized that stimulation of GABAA receptors by local infusion of muscimol into VP should produce the same effect as accumbens DA depletions (i.e. decreases in lever pressing should be accompanied by increases in chow intake). Based upon the results of experiment 1, additional control studies were performed. Experiment 2 studied the effect of VP injections of muscimol on free consumption and choice of the same two foods used in experiment 1. Experiment 3 assessed the behavioral effects of injections of muscimol into a control site dorsal to the VP. The 4th and final experiment involved injections of a retrograde tracer into VP or a dorsal control site in order to verify that the behaviorally active VP site received projections from the nucleus accumbens.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague–Dawley rats (Harlan Sprague Dawley; Indianapolis, IN, USA), weighing 275–300 g at the beginning of the experiments, were used for these studies (total n=62). All animals were housed in a colony with a 12-h light/dark cycle with lights on at 07:00 h, and temperature maintained at approximately 23 °C. Animals were initially food-deprived to 85% of their free feeding body weight, but then allowed modest growth over the course of the study (i.e. up to 95% of original weight) because the rats receive enough food in the operant chamber to allow for modest growth. Water was available ad libitum in the home cages at all times. Prior to surgery, animals were housed in pairs, and after surgery all animals were housed separately. Animal protocols were approved by the institutional animal care and use committee of the University of Connecticut, and the methods were in accord with the Guide for the Care and Use of Laboratory Animals. These experiments were conducted so as to minimize the number of animals used and any potential suffering.
Concurrent lever pressing/feeding behavioral procedures
Behavioral sessions were conducted in operant conditioning chambers (28 cm×23 cm×23 cm; Med Associates, Georgia, VT, USA) during the light period. Animals were trained in 30 min sessions, 5 days per week. In the first week of training, all rats were trained to lever press for 45 mg pellets (Research Diets, Inc., New Brunswick, NJ, USA) on a FR 1 schedule. In the second week, animals were shifted to a FR 5 schedule, which was maintained for 3–4 weeks to ensure stable performance. Subsequently, all rats were trained on the concurrent FR 5/chow feeding procedure. For this procedure, weighed amounts of laboratory chow (Prolab, Laboratory Diet, Brentwood, MO, USA; typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the 30 min FR 5 sessions. At the end of the session, rats were immediately removed from the chambers. Food intake was determined by weighing the remaining food, including spillage, while lever pressing was recorded by a computer program.
Operant pellet/laboratory chow food consumption task
Free feeding choice sessions were conducted in plastic tub cages (45 cm×24 cm×20 cm). Animals were trained in 30-min sessions, 5 days per week, during the light period. For the initial training, animals had free access to weighed amounts (20 g) of 45 mg sucrose operant pellets. After 4 weeks of training with only the operant pellets, weighed amounts of laboratory chow (15–20 g) were introduced to the apparatus. For this phase of the training, rats were allowed to choose between the operant pellets and the laboratory chow. Food consumption was determined by weighing each type of food separately both before and after the sessions.
Pharmacological agents
Muscimol hydrobromide was purchased from Sigma Chemical (St. Louis, MO, USA) and dissolved in 0.9% saline. Experiments 1 and 2 used three drug treatment conditions (saline vehicle, 5.0 ng, and 10.0 ng muscimol) in each of the two sites. Experiment 3 used two treatment conditions (saline vehicle and 10.0 ng muscimol). The doses of muscimol used were based upon pilot studies, and were intended to be very low so they did not have non-specific effects on a wide variety of behaviors.
Surgical and intracranial injection procedures
Rats were anesthetized with a solution (1.0 ml/kg, i.p.) that contained ketamine (100 mg/ml) and xylazine (0.75 ml of a 20 mg/ml solution added to 10.0 ml of ketamine solution), and placed in a stereotax. The incisor bar on the stereotax was set to 5.0 mm above the interaural line. All animals received bilateral implantations of stainless steel guide cannulae (25 gauge, extra-thin wall). For the VP site, guide cannulae were implanted 1.0 mm dorsal to target at the following coordinates: AP +0.8 mm (from bregma), ML ±2.5 mm (from midline), and DV −7.5 mm (from the skull surface). For the dorsal control site, guide cannulae were implanted at the following coordinates: AP +0.8 mm (from bregma), ML ±2.5 mm (from midline), and DV −5.0 mm (from the skull surface). The guide cannulae were secured to the skull with stainless steel screws and cranioplastic cement. To maintain patency of the cannulae prior to injection, stainless steel stylets were inserted. All animals were housed in separate cages after surgery, and were allowed 7–10 days to recover. After recovering from cannulae implantation surgery, rats in experiments 1 and 3 resumed training on the concurrent FR 5/chow feeding procedure for two additional weeks. Rats in experiment 2 resumed training on the free feeding choice procedure to two additional weeks. For bilateral intracranial (IC) injections through the cannulae, 30 gauge stainless steel injectors were used. The injectors were set to extend 1.0 mm beyond the tip of the guide cannulae. The injectors were connected to 10.0 μl Hamilton syringes with PE-10 tubing, and the injections were driven by a syringe pump (Harvard Apparatus) at a rate of 0.5 μl/min. Each side received 0.5 μl total volume, and the injectors were left in place for an additional 1 min to allow diffusion of the drug. Each animal received an injection of only one drug treatment condition.
Experiments
Four experiments were conducted.
Experiment 1: Effect of VP muscimol on concurrent FR 5/chow feeding procedure
Rats (n=25) were trained on the concurrent FR 5/chow feeding procedure as described above. After training, rats were implanted with cannulae in the VP, and training resumed after the postsurgical recovery period. Drug testing was conducted using a between-groups design, with each rat receiving only one drug treatment. Rats were randomly assigned to receive intracranial injections of saline vehicle, 5.0 ng muscimol, or 10.0 ng muscimol per side, in 0.5 μl total volume (see above). Directly following the injection procedure, the animals were placed in the operant chambers for a 30-min FR 5/chow feeding session. Behavioral measures included the total number of lever presses and the total amount of chow consumed.
Experiment 2: Effect of VP muscimol on consumption of operant pellets and laboratory chow
Rats (n=13) were trained on the operant pellet/chow feeding procedure, and then were implanted with cannulae in the VP. Behavioral training resumed after the postsurgical recovery period. Rats were randomly assigned to receive intracranial injections of either saline vehicle or 10.0 ng muscimol per side, in 0.5 μl total volume, with each rat receiving only one drug treatment, as described above. Immediately following the injection procedure, rats were placed in the feeding chambers and allowed to choose freely between operant pellets and the laboratory chow. Food consumption was determined by weighing each type of food separately both before and after the testing session.
Experiment 3: Effect of muscimol injections into the dorsal control site: Concurrent FR 5/chow feeding procedure
Rats (n=22) were trained on the concurrent FR 5/chow feeding procedure. After training, rats were implanted with cannulae in the dorsal control site, and training resumed after post surgical recovery. The drug injections, behavioral procedures and measures for this experiment were identical to those used in experiment 1.
Experiment 4: Retrograde tracer studies
The retrograde tracer cholera toxin B subunit (CTB 488, Molecular Probes, Invitrogen Corporation, Carlsbad, CA, USA) was injected into the VP (n=1) or a dorsal control site (n=1) of two different rats 1 week before perfusion. The injections of CTB were made with a glass micropipette and injected into the VP (AP: −0.8 mm, ML: ±2.6 mm, DV: −7 mm from dura; incisor bar on the stereotax was set between 3.2 and 4 mm below the interaural line) or the control site 2.0 mm dorsal to the VP. One week after tracer delivery, animals were anesthetized with CO2 and perfused with physiological saline followed by 3.7% formaldehyde. Tissue sections (40 μm) were sliced on a vibratome and stored in phosphate-buffered saline (PBS) at 4 °C until histological processing.
CTB visualization
Transported CTB was visualized using a standard immunohistochemistry protocol modified for the detection of CTB in free-floating sections. A series of free floating sections was rinsed for 30 min in 0.1 M PBS and endogenous peroxidase activity was quenched with a 20 min incubation in 0.5% hydrogen peroxide (H2O2)+1% methanol (MEOH) solution in 0.1 M PBS. Sections were rinsed in 0.1 M PBS (30 min) and permeablized and blocked for 1 h in a PBS solution containing 0.2% Triton+2.5% normal goat serum (NGS; Vector Laboratories Inc; Burlingame, CA, USA). Sections were then incubated in a solution of primary antibody rabbit anti-CTB (1:5000 Sigma-Aldrich)+2.5% NGS in 0.1 M PBS overnight at 4 °C. Following a 30 min rinse in 0.1 M PBS, the sections were incubated in secondary antibody biotinylated goat-anti-rabbit (1:200 Vector Laboratories Inc.)+2.5% NGS in 0.1 M PBS for 2 h at room temperature. After another 30 min rinse, the sections were then incubated in an avidin– biotin solution+0.1% Triton in 0.1 M PBS (ABC kit; Vector Laboratories Inc.) for 1 h at 20 °C followed by a 30 min rinse in 0.1 M PBS. Sections were then processed for immunoperoxidase reactivity using a diaminobenzidine (DAB) solution. The final reaction product was obtained using a DAB substrate kit for peroxidase with nickel (Vector Laboratories Inc.), using a 2–10 min processing at room temperature. Tissue was rinsed a final time for 30 min in 0.1 M PBS and mounted on slides to dry overnight. The mounted tissue was then cleared with Citrisolv and coverslipped using DPX (VWR International Ltd., Poole, UK).
Nissl staining procedures
After experiments 1–3 were completed, each animal was anesthetized with CO2 and transcardially perfused with physiological saline followed by a 3.7% formaldehyde solution. The brains were removed and stored in formaldehyde for several days (for post-fixation) and were then sliced and mounted on slides. Following slicing and mounting, slides were stained with Cresyl Violet, and cannula placements were determined using a microscope. All animals used for statistical analyses in the present experiments had verified cannula placements (14.9% of all implantations were rejected). Cannula placements for all animals receiving the 10 ng dose in experiments 1 and 3 are shown in Fig. 1 (see Pellegrino et al., 1979 for atlas reference pages).
Fig. 1.
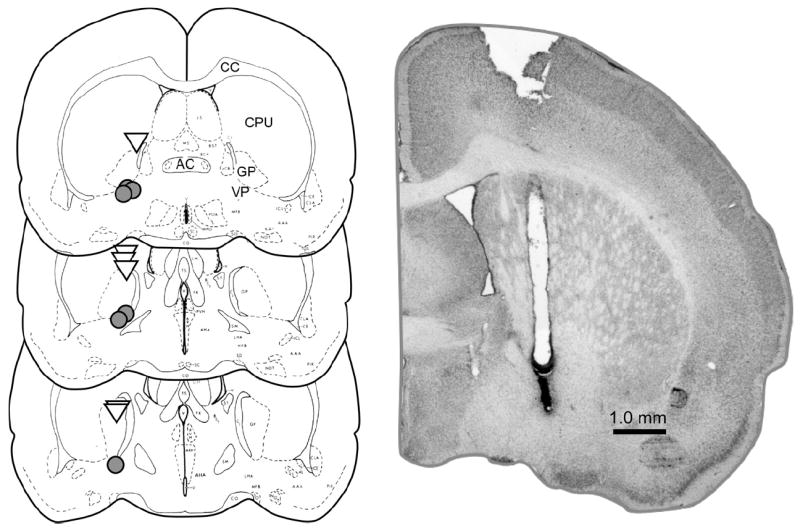
Left: Drawings indicating cannula placements for all animals that received 10.0 ng muscimol in the VP in experiment 1 (gray circles), and in the dorsal control site in experiment 3 (open triangles). For each animal, placements are only shown for one hemisphere. Figure is adapted from Pellegrino et al. (1979). Right: Composite photomicrograph of Nissl-stained section indicating representative VP cannula placement. CC =corpus callosum, CPU=caudate putamen, AC=anterior commissure, GP=globus pallidus.
Statistical analyses
Data for experiments 1 and 3, including total lever presses and chow intake quantities, were analyzed using a one-factor (drug dose, three levels) between subjects analysis of variance (ANOVA). If the overall ANOVA revealed a significant difference between treatment conditions, post hoc Dunnett tests were used to determine the source of the difference, with the vehicle-treated rats designated as the control group. Additionally, correlational analyses were used to measure the relationship between lever pressing and chow consumption. For experiment 2, pellet and chow consumption were separately analyzed using independent-sample t-tests and non-parametric analyses.
RESULTS
Experiment 1: Effect of VP muscimol on concurrent FR 5/chow feeding procedure
The results of experiment 1 are shown in Fig. 2. Infusion of muscimol into the VP dose-dependently reduced lever pressing [F(2, 24)=25.537; P<0.05] and increased chow consumption [F(2, 24)=10.484; P<0.05]. Dunnett tests revealed that both the 5.0 and 10.0 ng doses of muscimol reduced lever pressing and increased chow consumption. Furthermore, there was a significant inverse correlation between lever pressing and chow consumption in rats that received 5.0 and 10.0 ng muscimol (r=−0.620, df=14; P<0.05).
Fig. 2.
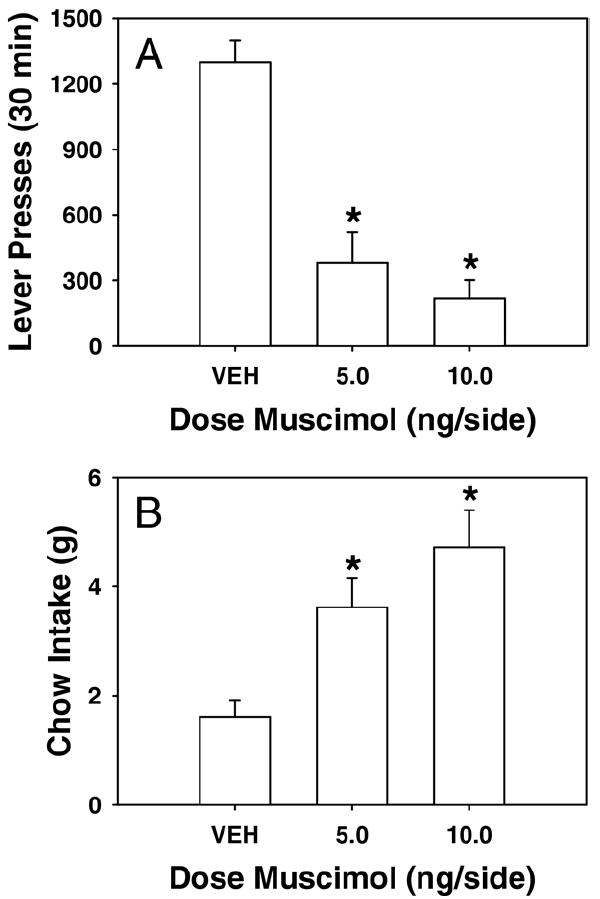
The effects of VP injections of muscimol on performance of the concurrent lever pressing/chow feeding choice procedure. Rats received treatment with either saline vehicle, 5.0 ng or 10.0 ng muscimol. (A) Mean (±S.E.M.) number of lever presses (FR 5 schedule) during the 30 min session. (B) Mean (±S.E.M.) gram quantity of chow intake. (* P<0.05, different from vehicle.)
Experiment 2: Effect of VP muscimol on consumption of operant pellets and laboratory chow
Animals treated with saline vehicle (n=8) consumed 18.15±0.40 g (mean±S.E.M.) of operant pellets and 0.59±0.23 g of laboratory chow, whereas animals that received 10.0 ng muscimol in the VP consumed 14.34±1.98 g of operant pellets and 0.12±0.08 g of laboratory chow. Although there was a tendency for animals treated with 10.0 ng muscimol into the VP to show a slight suppression of both operant pellet and chow intake relative to those that received vehicle treatment, analyses with either parametric (t-test, separate variances) or non-parametric (Mann-Whitney U) tests indicated that there was not a statistically significant difference between groups (P>0.05).
Experiment 3: Effect of muscimol injections into the dorsal control site: Concurrent FR 5/chow feeding procedure
The results of experiment 3 are presented in Fig. 3. Infusion of muscimol into a dorsal control site failed to produce a statistically significant effect on the total number of lever presses [F(2, 21)=0.69; P=0.5] or chow consumption [F(2, 21)=1.47; P=0.25 Furthermore, there was no significant inverse correlation between lever presses and chow consumption (r=−.049, df=14; P=0.8) in rats that received 5.0 and 10.0 ng muscimol.
Fig. 3.
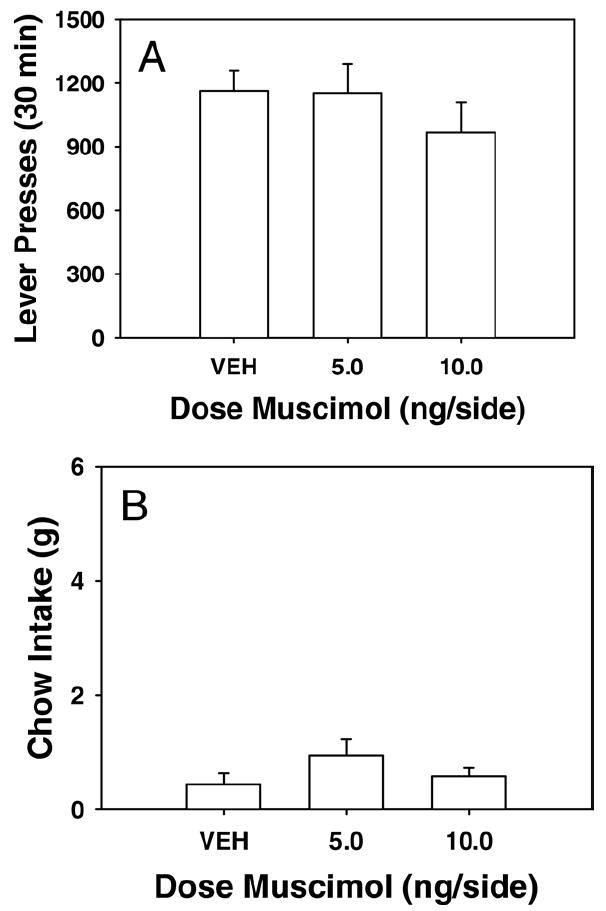
The effects of injections of muscimol into the dorsal control site on performance of the concurrent lever pressing/chow feeding choice procedure. Rats received treatment with either saline vehicle, 5.0 ng or 10.0 ng muscimol. (A Mean (± S.E.M.) number of lever presses (FR 5 schedule) during the 30 min session. (B) Mean (±S.E.M.) gram quantity of chow intake.
Experiment 4: Retrograde tracer studies
As shown in Fig. 4, injections of CTB into the VP resulted in retrograde labeling of neurons in ventral striatal areas surrounding the anterior commissure, including nucleus accumbens core and adjacent areas of anteroventromedial neostriatum. In contrast, injections of CTB dorsal to the VP, in the vicinity of the globus pallidus and overlying neostriatum, failed to label the nucleus accumbens, and instead labeled cells in the dorsomedial neostriatum.
Fig. 4.
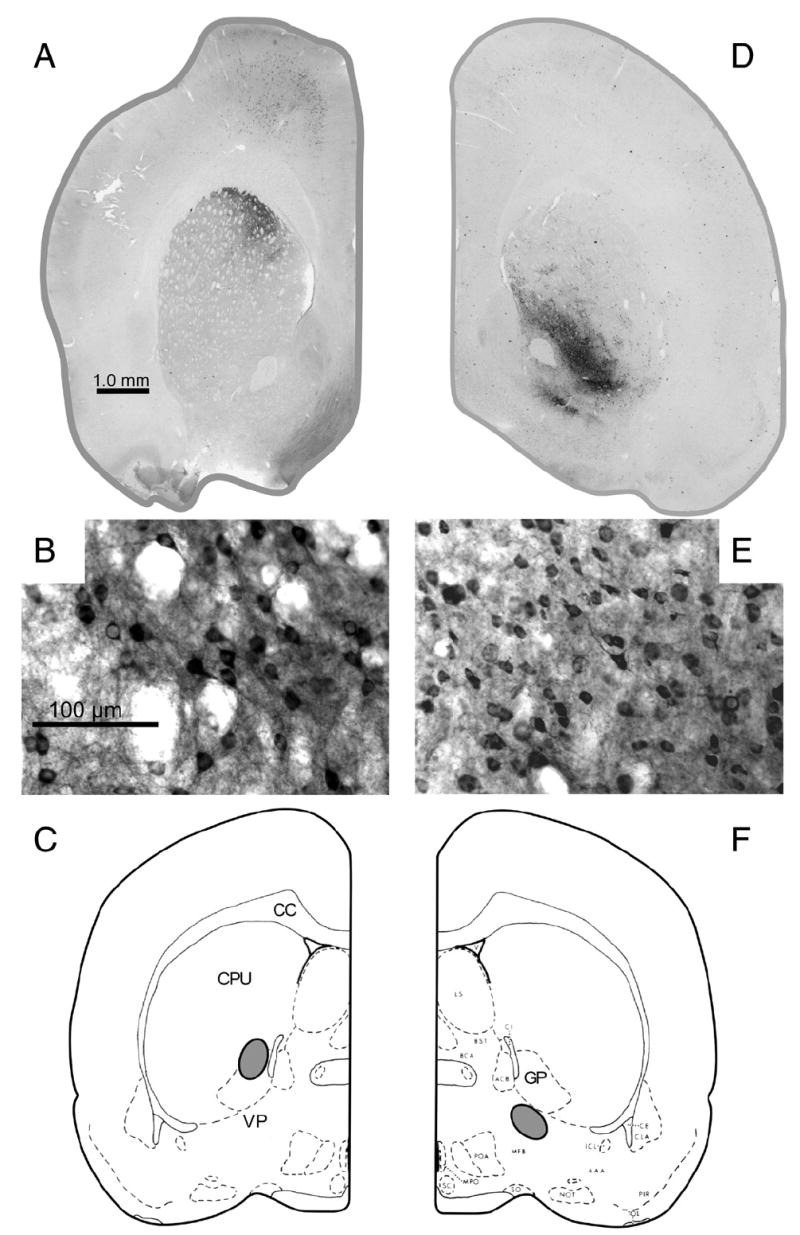
Results of the retrograde tracing experiment, showing retrograde labeling following injections of CTB into the VP and control areas dorsal to the VP. (A–C) Labeling of dorsomedial neostriatum after injection into globus pallidus and neighboring neostriatum. (A) Low magnification photomicrograph of labeled region in neostriatum. (B) High magnification photomicrograph, showing CTB-positive cell bodies. (C) Injection site (gray oval). (D–F) Labeling of ventral striatal regions after injection into VP. (D) Low magnification photomicrograph of labeled region in ventral striatal areas surrounding the anterior commissure, including nucleus accumbens core and adjacent areas of anteroventromedial neostriatum. (E) High magnification photomicrograph, showing CTB-positive cell bodies. (F) Injection site (gray oval). C and F were modified from Pellegrino et al. (1979).
DISCUSSION
The present results indicate that VP GABA is involved in modulating response allocation in food-seeking behavior. Injections of the GABAA agonist muscimol into the VP produced behavioral effects similar to those previously shown to be induced by interference with DA transmission in nucleus accumbens. With animals responding on a concurrent lever pressing/chow feeding task, muscimol produced a dose-related decrease in lever pressing that was accompanied by an increase in chow intake. In muscimol-treated rats, there was a robust inverse correlation between lever pressing and chow intake. These effects were not produced by injections of muscimol into a control site dorsal to VP. Moreover, VP injections of muscimol did not alter preference between operant pellets and chow in free-feeding choice tests. In view of data indicating that VP receives projections from nucleus accumbens, the present findings, together with other studies, indicate that nucleus accumbens and VP are components of the brain circuitry regulating response allocation in food-seeking and effort-related choice behavior.
The results of experiment 1 clearly show that VP injections of muscimol produce a dose-dependent decrease in lever pressing and a corresponding increase in chow consumption in the operant concurrent choice procedure. Importantly, this pattern of results closely resembles the effects of both nucleus accumbens DA depletions and intra-nucleus accumbens administration of DA antagonists, in that stimulation of GABAA receptors in the VP, as well as interference with nucleus accumbens DA transmission, both shift behavior from lever pressing to chow intake with animals performing on this task (Cousins and Salamone, 1994; Cousins et al., 1993; Koch et al., 2000; Nowend et al., 2001; Salamone et al., 1991; Sokolowski and Salamone, 1998). The shift from lever pressing to chow intake seen after administration of muscimol into the VP does not appear to be dependent upon a drug-induced alteration of food preference, because experiment 2 showed that muscimol injections did not alter preference between operant pellets and laboratory chow. Thus, consistent with the observations made in previous studies with dopaminergic manipulations, the present results indicate that rats treated with a low dose (i.e. 5.0–10.0 ng) of muscimol in the VP remain directed toward the acquisition and consumption of food. Although lever pressing is reduced by stimulation of VP GABAA receptors, muscimol-treated rats show alterations in the allocation of their instrumental responding, and select a new path to an alternative food source (i.e. the concurrently available chow). This pattern of results suggests that rats injected with muscimol into the VP, like rats with impaired accumbens DA transmission, show alterations in their effort-related choice behavior. Additional studies involving other behavioral tasks (e.g. T-maze; Salamone et al., 1994; Cousins et al., 1996; ratio schedules, Salamone et al., 2001; Correa et al., 2002; Mingote et al., 2005) will be necessary to provide a more complete characterization of the effort-related functions of VP GABA.
In addition to the operant concurrent choice procedure, the relationship between nucleus accumbens DA transmission and VP GABA transmission has been observed using other behavioral procedures. For example, prepulse inhibition of the acoustic startle response, which is known to be affected by dopaminergic manipulations of the nucleus accumbens, also is modulated by VP GABA transmission. Deficits in prepulse inhibition were produced by VP infusions of the GABAA antagonist picrotoxin, but not by the GABAB antagonist saclofen (Swerdlow et al., 1990; Kodsi and Swerdlow, 1995). In addition, the deficits in prepulse inhibition that were induced by stimulation of DA receptors in accumbens were reversed by VP infusions of the GABAA agonist muscimol (Swerdlow et al., 1990; Kodsi and Swerdlow, 1995). Novelty-induced locomotion was reduced by VP injections of the GABAA agonist muscimol (Austin and Kalivas, 1990; Hooks and Kalivas, 1995). Conversely, blockade of GABAA receptors with either the competitive inhibitor bicuculline or the non-competitive inhibitor picrotoxin stimulated locomotor activity (Mogenson and Nielsen, 1983; Austin and Kalivas, 1990). Inactivation of the VP also has been shown to affect working memory and foraging choice (Floresco et al., 1999). In summary, the present results are consistent with previously reported findings that describe the functional relationship between nucleus accumbens and VP.
In addition to studying prepulse inhibition and locomotion, other studies have examined the function of VP GABAA transmission in aspects of feeding behavior. It has been reported that VP infusions of the GABAA antagonist, bicuculline, can increase feeding in rats (Smith and Berridge, 2005; Stratford et al., 1999). More recently, Shimura et al. (2006) reported that bicuculline injections in the VP produced a substantial and selective increase in consumption of a saccharin solution compared with water or quinine solutions. The behavioral processes involved in these effects are uncertain, and the notion that GABAA blockade in the VP could be mediating hedonic reactivity remains somewhat unclear; neither Smith and Berridge (2005) nor Shimura et al. (2006) reported a bicuculline-induced increase in hedonic taste reactivity. Nevertheless, it was reported that VP muscimol injections not only significantly suppressed consumption of saccharin, water, and quinine solution, but that muscimol treatment also significantly increased aversive taste reactivity responses to all solutions tested (Shimura et al., 2006). In light of the findings described above, it has been argued that facilitation of GABAA neurotransmission in the VP regulates feeding behavior through a mechanism involved in mediating aversive responses to food, but blockade of GABAA receptors in the VP does not produce the opposite (i.e. hedonic) response. In experiment 2 of the present study, there was a tendency for consumption of both types of food to be slightly suppressed in rats treated with 10.0 ng muscimol; however, this effect was not statistically significant. Moreover, this dose of muscimol did not produce a shift in food preference in the free choice procedure (experiment 2), suggesting that the shift in behavior observed in experiment 1 was not due to increased aversive taste reactivity. Also, it seems unlikely that producing a general food aversion effect would lead to an increase in chow intake. Recent studies from our laboratory have shown that the cannabinoid CB1 inverse agonist AM251, which is known to produce food aversions (McLaughlin et al., 2005), does not produce a shift from lever pressing to chow intake with rats responding on the concurrent choice task (Sink et al., 2007). Rats treated with doses of AM251 that induced conditioned gaping, which is a sign of nausea or malaise in rats, showed suppressed lever pressing but no corresponding increase in chow intake when tested in the concurrent choice task (Sink et al., 2007). Finally, in considering the relation between the present results and those of Shimura et al. (2006), it is worthwhile to emphasize the fact that the highest dose of muscimol used in the present studies was 10.0 ng, while the dose that produced aversive responses in the Shimura et al. (2006) study was 10 times higher (i.e. 100.0 ng).
Experiment 3 demonstrated that the shift in choice behavior observed in experiment 1 was due to stimulation of GABAA receptors in the VP, but not in sites dorsal to the VP, including globus pallidus and caudate-putamen. This type of control experiment is important, because it demonstrates some degree of site specificity, and emphasizes that the results of experiment 1 were not due to non-specific consequences of simply injecting muscimol into the forebrain. In accordance with the results of experiment 3, experiment 4 demonstrated that the behaviorally active site, at which muscimol produced the shift in choice, receives projections from nucleus accumbens, whereas the dorsal control site used in experiment 3 receives projections from neostriatal regions dorsal to the accumbens. Although the present studies indicate some degree of site specificity in the effects of VP muscimol injections, they do not provide a comprehensive map of the effective brain region, and future experiments need to explore both the medial–lateral and dorsal–ventral extent dimensions in order to provide a more precise mapping of this effect.
CONCLUSIONS
In summary, the present findings serve to support the hypothesis that nucleus accumbens DA transmission and VP GABA transmission form part of functional circuit that underlies various behavioral processes, including prepulse inhibition, locomotion, and effort-related choice. The brain circuitry regulating effort-related choice also appears to include several additional structures, including prefrontal/anterior cingulate cortex and basolateral amygdala (Walton et al., 2002, 2003; Schweimer et al., 2005; Schweimer and Hauber, 2006; Floresco and Ghods-Sharifi, 2007; Salamone et al., 2006, 2007). Further experimentation will be needed to characterize more completely the contribution that these structures make to effort-related processes, and to understand the relation between the basic neuroscience research in this area and clinical studies of energy-related disorders such as psychomotor slowing, anergia and fatigue (Salamone et al., 2006, 2007).
Acknowledgments
This research was supported by a grant to J.D.S. from the United States NIH/NIMH. Laura Font was supported by a grant of Fundació Caixa Castelló-Bancaixa.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DAB
diaminobenzidine
- FR
fixed ratio
- NGS
normal goat serum
- PBS
phosphate-buffered saline
- VP
ventral pallidum
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther. 1990;252:1370–1377. [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci. 2004;20:593–596. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- Chang HT, Tian Q, Herron P. GABAergic axons in the ventral forebrain of the rat: an electron microscopic study. Neuroscience. 1995;68:207–220. doi: 10.1016/0306-4522(95)00109-v. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm Gen Sect. 1993;93:123–143. doi: 10.1007/BF01245342. [DOI] [PubMed] [Google Scholar]
- Collier GH, Jennings W. Work as a determinant of instrumental performance. J Comp Physiol Psychol. 1969;68:659–662. [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology (Berl) 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology (Berl) 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann N Y Acad Sci. 1999;877:711–716. doi: 10.1111/j.1749-6632.1999.tb09308.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp 1301565. in press. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Heimer L, Switzer RD, Van Hoesen GW. Ventral striatum and ventral pallidum: Components of the motor system? Trends Neurosci. 1982;5:83–87. [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbens-pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64:587–597. doi: 10.1016/0306-4522(94)00409-x. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Parente MA, Stellar JR. NMDA-induced lesions of the nucleus accumbens or the ventral pallidum increase the rewarding efficacy of food to deprived rats. Brain Res. 1996;722:109–117. doi: 10.1016/0006-8993(96)00202-8. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection subserving ambulatory activity. Am J Physiol. 1980;238:R65–R69. doi: 10.1152/ajpregu.1980.238.1.R65. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology (Berl) 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Res. 1995;684:26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD. Functional aspects of the ventral pallidum. Amino Acids. 2000;19:201–210. doi: 10.1007/s007260070050. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. New York: Plenum; 1979. [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology (Berl) 1992;107:160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiatry Rev. 2006;2:267–280. [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Mem. 2006;13:777–782. doi: 10.1101/lm.409306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0988-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63:7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology (Berl) 1990;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14:139–151. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- van den Bos R, van der Harst J, Jonkman S, Schilders M, Spruijt B. Rats assess costs and benefits according to an internal standard. Behav Brain Res. 2006;171:350–354. doi: 10.1016/j.bbr.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res. 2004;154:19–30. doi: 10.1016/j.bbr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM, Rushworth MF. Calculating the cost of acting in frontal cortex. Ann N Y Acad Sci. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]


