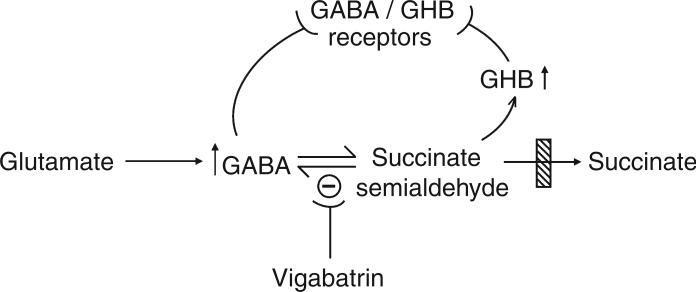
SIR–Succinic semialdehyde dehydrogenase (SSADH; aldehyde dehydrogenase 5a1, Aldh5a1; OMIM 271980, 610045) deficiency is the most prevalent disorder of γ-aminobutyrate (GABA) metabolism, and one in which two neuroactive compounds (GABA and γ-hydroxybutyrate [GHB]) accumulate (Fig. 1).1,2 The clinical phenotype is that of a static encephalopathy, including global delay in development, hypotonia, ataxia, poorly-developed or absent speech, sleep disturbance, and seizures.2 Since SSADH deficiency lacks the usual concomitants (hyperammonemia, hypoglycemia, metabolic acidosis) of other life-threatening organic and amino acid disorders, the lifespan of patients is not thought to be truncated, and parents have realistic concerns as to the evolution of the disease phenotype with age.
Figure 1.
Schematic diagram of GABA synthesis and degradation in the central nervous system (not all sources of GABA are shown). The site of the block in heritable succinate semialdehyde dehydrogenase deficiency is depicted by the crosshatched box. T-shaped bars indicate receptor interactions or sites of enzyme inhibition. Vigabatrin is an irreversible inhibitor of GABA-transaminase (shown by the – sign), a common intervention in SSADH deficiency. GABA, γ-aminobutyric acid; GHB, γ-hydroxybutyric acid); SSADH, succinic semialdehyde dehydrogenase.
Accumulation of GABA and GHB are unique features of SSADH deficiency, and they have pathophysiological implications. GABA, the main inhibitory transmitter in mammals, has a number of receptor systems, and alterations of these receptors have been documented both in Aldh5a1−/− mice and in patients.3-6 Metabolic studies in Aldh5a1−/− murine embryos have revealed significant increases in both GABA and GHB as early as E10 (embryo day of life 10), which is of interest since GABA is excitatory during development and might predispose neural circuity to a hyperexcitatory state.7 GHB also has its own receptor systems, and its accumulation may increase GABA secondarily while exacerbating the effects of GABA on different receptors.8 Recently, GABA(A) and GABA(B) receptor abnormalities have been suggested in SSADH-deficient patients through studies employing [11C]flumazenil binding and transcranial magnetic stimulation.4,5 Here we report the case history and follow-up for one of the earliest-diagnosed patients with SSADH deficiency.
The patient, a female, was originally evaluated at 9 years 2 months of age. Parental consent was provided. Her developmental history had shown early delays in gross motor and language development. She was hypotonic in infancy and did not develop good head control until 6 months of age. She first walked alone at 18 months. Language skills were very delayed; her first meaningful words were at 4 years of age. Her vocabulary at 9 years was limited to 12 words, placing her expressive language skills at approximately a 16-month level. She was able to sign several words with a signing level of approximately 2 years of age. Receptive language skills were assessed as being at a 4 to 4.6 year level. Since approximately 8 years of age athetoid dystonic movements had been noted, affecting both arms with head turning to the right and intermittent mouth opening associated with jaw deviation to the right.
On general examination the most striking feature was extreme hyperactivity. There was also a marked attention deficit. She could understand and follow simple commands. Cranial nerve examination was normal except for a loss of optokinetic nystagmus when the tape was brought in from the left; fundi and disks were normal; peripheral sensation was intact to touch and pin-prick. Mild hypotonia was present, but muscle strength was normal. Appendicular and truncal coordination appeared normal. Deep tendon reflexes were symmetrical and equal at 1+ and plantar responses were flexor. Examination of the gait revealed a tendency for the right arm to be extended and posteriorly positioned in an athetoid dystonic posture. Diplegic scissoring was apparent with more inturning of the right than left leg.
Abnormal movements (potentially related to thioridazine [15 mg p.o. tid] intervention to control behavior) were brief and poorly sustained, and associated with a mask-like facies absent earlier at 6 years of age. Magnetic resonance imaging of the head showed normal Tl, T2, and balanced-pulse sequences. Abnormal excretion of GHB had been detected at 6 years of age and SSADH deficiency was documented in peripheral blood leucocytes at that time.9
At age 29 years our patient is cared for in adult day-care settings. Cognitive function is globally delayed, but hyperactivity has remitted. Current problems include fluctuating levels of depression, frustration/aggression, and repetitive behavior. These observations emanate from caregivers, since there is not a specialist involved in her routine care. She receives no medication. Her primary caregiver supplies herbal products including acidophilus, and St. John's wort for depression. She receives a daily multivitamin preparation containing folate and vitamin B12. She communicates via sign-language. Sleep schedule is normal; occasional extended sleep periods last 12 to 15 hours. Overall, she is described as an affectionate individual, who is both active (enjoying horseback riding, swimming, and bowling) and healthy.
The primary neurobehavioral features associated with adolescent and adult SSADH deficiency include attention deficit, hyperactivity, anxiety, obsession-compulsion, aggressive behavior, hallucinatory episodes, and autistic features.10,11 For our patient, significant hyperactivity in adolescence evolved into moderate psychopathology in adulthood, comprised of depression and obsession-compulsion, although neither were debilitating. The phenotype for our patient in adulthood is similar to that seen in other patients. Her lack of neurological deterioration over a 20-year period reinforces the concept that the phenotype of SSADH deficiency is a static encephalopathy. To our knowledge, this is the first extended follow-up of this disorder from childhood into adulthood.
Acknowledgements
Supported in part by NIH RR OO827, NS 23876, HD 04608, and NS 40270, and a grant from the Pediatric Neurotransmitter Disease Association. The nursing staff of the Clinical Research Center of the UCSD Medical Center is gratefully acknowledged for their assistance in all clinical investigations. We thank the parents and caregivers of our patient for their support in the development of this work.
References
- 1.Gibson KM, Jakobs C, Pearl PL, Snead OC. Murine succinate semialdehyde dehydrogenase (SSADH) deficiency, a heritable disorder of GABA metabolism with epileptic phenotype. IUBMB Life. 2005;57:639–44. doi: 10.1080/15216540500264588. [DOI] [PubMed] [Google Scholar]
- 2.Pearl PL, Taylor JL, Trzcinski S, Sokohl A. The pediatric neurotransmitter disorders. J Child Neurol. 2007;22:606–16. doi: 10.1177/0883073807302619. [DOI] [PubMed] [Google Scholar]
- 3.Buzzi A, Wu Y, Frantseva MV, et al. Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 4.Pearl P, Taylor J, Trzcinski S, et al. 11C Flumanzenil PET imaging in patients with SSADH deficiency. Brain. Forthcoming. [Google Scholar]
- 5.Reis J, Cohen L, Pearl P, et al. Transcranial magnetic stimulation reveals altered cortical excitability in succinic semialdehyde dehydrogenase deficiency. Epilepsia. Forthcoming. [Google Scholar]
- 6.Wu Y, Buzzi A, Frantseva M, et al. Status epilepticus in mice deficient for succinate semialdehyde dehyrogenase: GABAA receptor-mediated mechanisms. Ann Neurol. 2006;59:42–52. doi: 10.1002/ana.20686. [DOI] [PubMed] [Google Scholar]
- 7.Jansen EEW, Struys EA, Jakobs C, et al. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. J Inherit Metab Dis. 2008;31(Suppl 1):137. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta AK, Gould GG, Gupta M, et al. Succinate semialdehyde dehydrogenase deficiency does not down-regulate gamma-hydroxybutyric acid binding sites in the mouse brain. Mol Genet Metab. 2006;88:86–9. doi: 10.1016/j.ymgme.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Gibson KM, Hoffmann G, Nyhan WL. 4-Hydroxybutyric aciduria in a patient without ataxia or convulsions. Eur J Pediatr. 1988;147:529–31. doi: 10.1007/BF00441983. [DOI] [PubMed] [Google Scholar]
- 10.Knerr I, Pearl PL, Bottiglieri T, Snead OC, Jakobs C, Gibson KM. Therapeutic concepts in succinate semialdehyde dehydrogenase (SSADH; ALDH5a1) deficiency (gamma-hydroxybutyric aciduria). Hypotheses evolved from 25 years of patient evaluation, studies in Aldh5a1−/− mice and characterization of gamma-hydroxybutyric acid pharmacology. J Inherit Metab Dis. 2007;30:279–94. doi: 10.1007/s10545-007-0574-2. [DOI] [PubMed] [Google Scholar]
- 11.Knerr I, Gibson KM, Jakobs C, Pearl PL. Neuropsychiatric morbidity in adolescent and adult succinic semialdehyde dehydrogenase deficiency patients. CNS Spectr. 2008;13:598–605. doi: 10.1017/s1092852900016874. [DOI] [PMC free article] [PubMed] [Google Scholar]