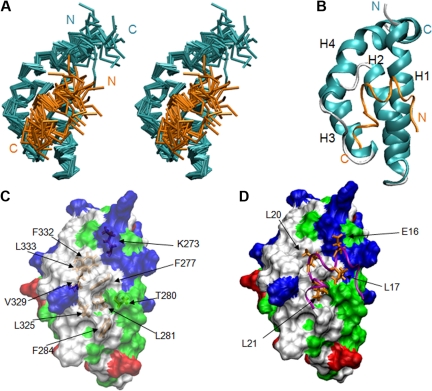
Figure 2.
Solution structure of the eTAFH domain–HEB peptide complex. (A) Stereoview of an ensemble of 20 lowest energy NMR solution structures. The backbone of residues G267 to Q353 of the eTAFH domain of AML1-ETO (turquoise) and residues I12 to M26 of the AD1 domain from HEB (orange) are displayed after superimposing the structures using residues Q269 to L350 of eTAFH and E16-L21 of HEB. (B) Ribbon representation of the lowest energy structure. (C) Surface of the eTAFH domain with the side chains of the binding site displayed and labeled (white indicate nonpolar residues; red, acidic residues; blue, basic residues; green, polar residues). (D) HEB backbone represented as a ribbon with the side chains interacting with eTAFH domain displayed and labeled. Vmd-Xplor was used to generate the figures.