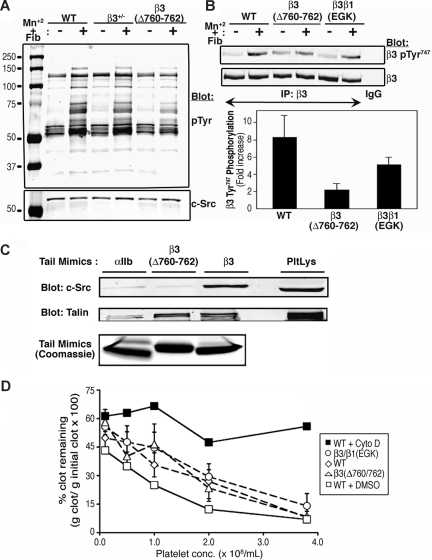
Figure 4.
Platelet tyrosine phosphorylation, fibrin clot retraction, and binding properties of β3 cytoplasmic domain mutants. (A) Platelets were incubated in suspension in the presence of 250 μg/mL fibrinogen plus or minus 0.5 mM MnCl2 for 20 minutes at room temperature and then lysed. Protein tyrosine phosphorylation was assessed by immunoblotting with antiphosphotyrosine antibodies (pTyr). Blots were stripped and reprobed with an antibody to c-Src. (B) Phosphorylation of β3 Tyr747 was detected with a phospho-specific antibody. Blots were reprobed with an antibody to total β3 and phosphorylation data were normalized for β3 expression. The bar graph depicts the fold-increase in β3 Tyr747 phosphorylation induced by MnCl2 + fibrinogen. Data are means (± range) for 2 independent experiments. (C) Pull-down of c-Src and talin from mouse platelet lysate (PltLys) by recombinant wild-type and mutant β3 cytoplasmic domain model proteins. An αIIb cytoplasmic domain protein was used as a negative control. Bound c-Src and talin were detected by immunoblotting. Loading of pull-down beads with recombinant cytoplasmic domains was monitored by staining with Coomassie brilliant blue. (D) Fibrin clot retraction using platelet-rich plasma from wild-type or mutant mice. Clotting was initiated with 9.4 U/mL thrombin and 1.9 mM CaCl2. Data represent mean (± SEM) for least 3 separate experiments performed in duplicate on at least 4 animals with each genotype. Experiments with wild-type platelets in the presence of 10 μM cytochalasin D (cyto D) or vehicle (dimethyl sulfoxide [DMSO]) were performed in duplicate on platelets from 4 animals to confirm that clot retraction was dependent on actin polymerization in this assay.