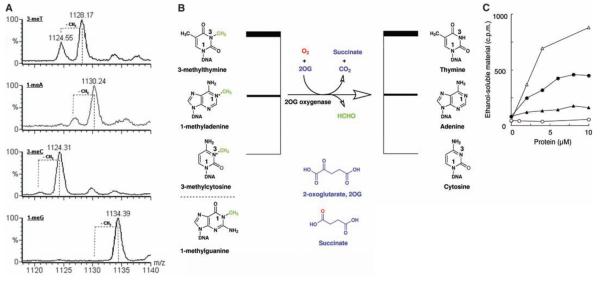
Fig. 3.
In vitro activity of Fto with ss-DNA substrates. (A) Demethylation of ss-DNA substrates by Fto. LC-MS data for the incubation of synthetic 15-nucleotide oligomers of the form 5′-TT(methylated base)TTTTTTTTTTTT-3′ with Fto, cofactors, and cosubstrates; positions of expected peaks for demethylated substrates are indicated by dotted lines. Smaller peaks at higher masses than reactant peaks probably arise from Na+ and K+ adducts of the methylated oligonucleotides. m/z = mass-to-charge ratio, shown in units of Dalton. (B) Stoichiometry of the Fto reaction. (C) Release of formaldehyde from methylated poly(dA) and poly(dT). FTO and ABH3 were assayed for demethylase activity by incubation with [14C]-methylated poly(dA) or [14C]-methylated poly(dT) [total counts per minute (c.p.m.) 1000 or 800, respectively] at 37°C for 15 min. Release of ethanol-soluble [14C]-formaldehyde was monitored. FTO -○-, -●-; ABH3 -∆-, -▲-. [14C]-methylated poly(dA) -○-, -∆-; [14C]-methylated poly(dT) -●-, -▲-. In the absence of 2OG, no significant activity was detected; the ethanol soluble material released by FTO or ABH3 was at background level (less than 100 c.p.m).