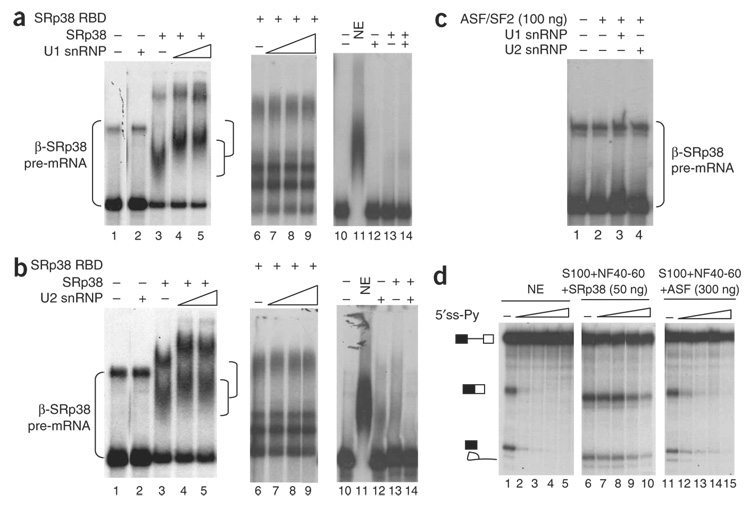
Figure 4.
SRp38 interacts with both U1 and U2 snRNP complexes on the SRp38 substrate. (a,b) Purified U1 snRNP (a) or U2 snRNP (b), GST-tagged SRp38 (150 ng) or GST-tagged SRp38 RBD (250 ng) were incubated with β-SRp38 pre-mRNA (lanes 1–9) or β-control pre-mRNA (lanes 10–14). Following addition of heparin (0.8 mg ml−1), complexes were resolved by 5% nondenaturing PAGE. Increasing amounts (300 ng and 600 ng) of U1 snRNP (a) or U2 snRNP (b) were incubated with SRp38 and β-SRp38 RNA (lanes 4, 5 and 6). The complexes formed are indicated with brackets. GST-tagged SRp38 RBD (250 ng) was incubated with β-SRp38 RNA (lane 6), and increasing amounts of U1 or U2 snRNPs (150 ng, 300 ng or 600 ng) were added (lanes 7–9). SRp38 (150 ng) was incubated with β-control pre-mRNA alone (lane 13) or β-control pre-mRNA plus U1 or U2 snRNPs (300 ng; lane 14). (c) His-tagged ASF/SF2 was incubated with β-SRp38 in the absence (lane 2) or in the presence of U1 snRNP (lane 3) or U2 snRNP (lane 4). (d) Splicing reactions were performed with the β-SRp38 RNA. The (5′ ss-Py) oligonucleotide was preincubated with nuclear extract (NE) or with S100 plus NF40–60 and SRp38 before splicing reactions were carried out. Final concentrations of the oligonucleotide are 0 µM, 0.1 µM , 0.5 µM, 1 µM and 2 µM. Note that 300 ng ASF/SF2 was used in the splicing reactions to achieve similar splicing levels as in the NE.