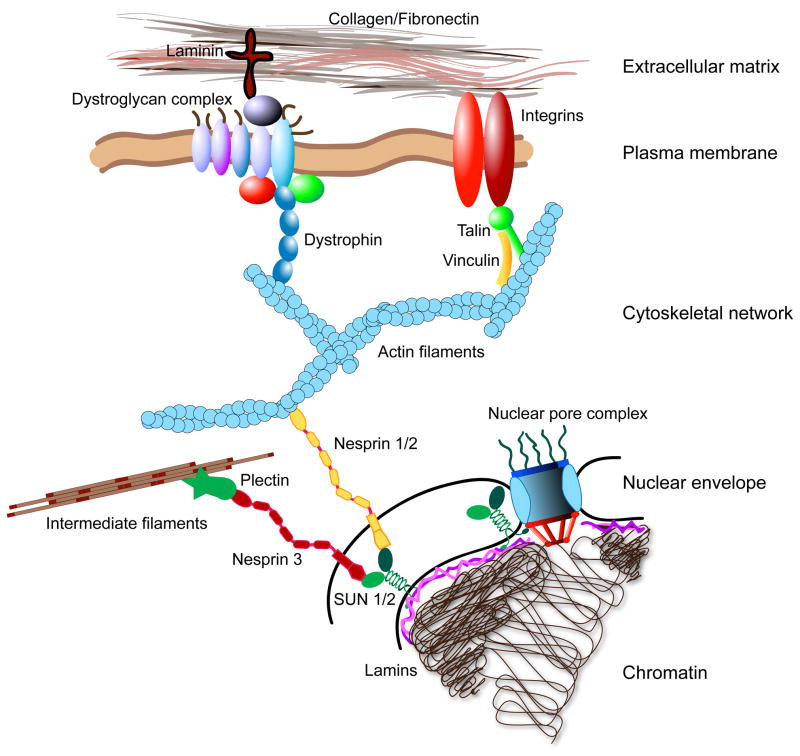
Figure 2. Force transmission between the extracellular matrix (ECM) and the nucleus.
Extracellular forces are transmitted through the ECM, consisting of tissue-specific proteins such as collagen, laminin, and fibronectin. Adhesion complexes at the cell surface physically link the ECM to the cytoskeleton. For example, focal adhesions, comprised of integrins, talin, vinculin, and other proteins, connect the ECM to actin filaments. In skeletal muscle, the dystrophin-associated protein complex links the ECM to actin filaments. The configuration and binding affinity of these complexes can be modulated through intra- and extracellular signalling. Intracellular forces are then transmitted through the cytoskeletal network (i.e., actin filaments, microtubules, and intermediate filaments). The cytoskeleton is coupled to the nucleus through nesprins and possibly other proteins on the outer nuclear membrane. The giant isoforms of nesprins-1 and -2 bind to actin filaments, whereas nesprin-3 can associate with intermediate filaments through plectin. Nesprins interact across the luminal space with inner nuclear membrane proteins (e.g., SUN1 and SUN2), that are retained there by interaction with other nuclear envelope proteins such as lamins and emerin84. Nuclear lamins and SUN proteins also bind to nuclear pore complexes, which could contribute to nuclear cytoskeletal coupling. Finally, lamins form stable nuclear structures and can bind DNA, thus completing the force transmission between the ECM and the nuclear interior. Mutations in any of these components, as well as changes in cellular structure and organization, or changes in the cellular environment, could disturb mechanotransduction signalling and result in altered cellular function; however, this has only been conclusively demonstrated for a subset of these molecules, motivating further research.