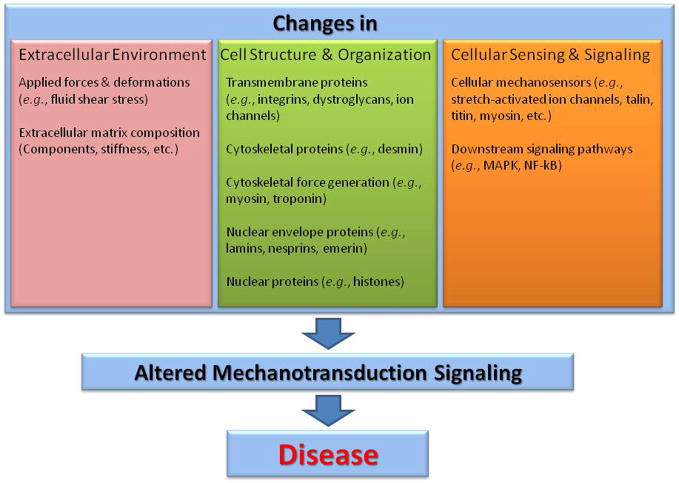
Figure 3. Unifying characteristics of mechanotransduction disorders.
Altered cellular mechanotransduction signalling can be caused by changes in the extracellular environment (e.g., variation in the mechanical forces or deformations experienced by the tissue, or changes in extracellular matrix composition that affect its stiffness and biochemical properties), the cellular structure and organization, or elements of the mechanotransduction process itself. Changes in cellular structure and organization often result from inherited or de novo mutations in proteins that are part of the force generating machinery, the cytoskeletal network, or the nuclear envelope and interior. This category also includes transmembrane proteins involved in cell-cell or cell-ECM adhesion. Abnormal function of these proteins can alter the intracellular force distribution and thus mechanotransduction signalling. In contrast, defects in the cellular mechanosensors can disturb mechanotransduction signalling even in the case of normal force distribution. Note that many proteins can fall into more than one category, as structural proteins could also have mechanosensing capabilities, and mechanotransduction signalling can in turn cause changes in cellular structure and organization and the extracellular environment. Importantly, mechanical activation often initiates multiple signalling pathways at once that can significantly overlap and cross-talk, making it more difficult to study specific pathways. Furthermore, several of the signalling pathways are often shared with “classical”, receptor-mediated pathways. For example, the mitogen-activated protein kinase (MAPK) pathway can be turned on by mechanical strain as well as by receptor-linked tyrosine kinases (e.g., epidermal growth factor receptor). Ultimately, excessive and prolonged disturbances in the normal mechanotransduction signalling can result in many disease conditions.