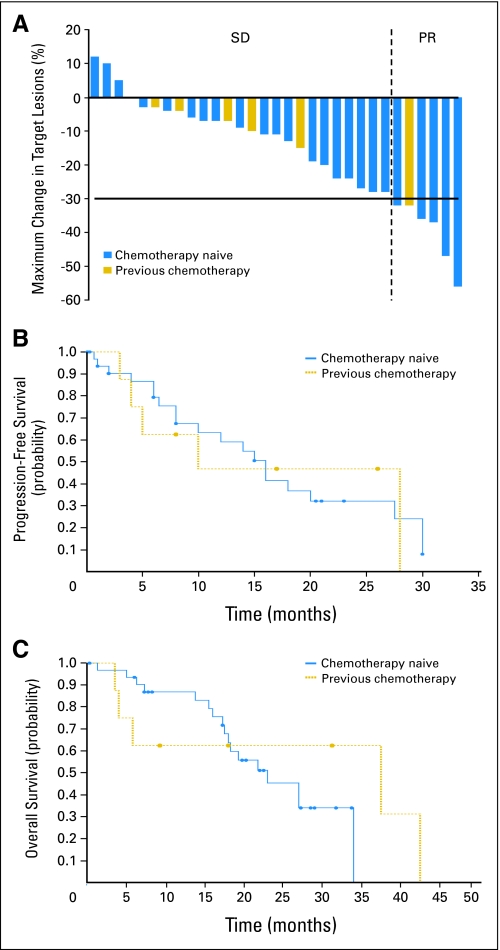
Fig 2.
(A) Maximum percentage of tumor reduction for target lesions for by Response Evaluation Criteria in Solid Tumors in all papillary thyroid cancer (PTC) patients who had stable disease (SD) or partial response (PR). With intent to treat analysis, six PRs (15%), 25 SDs (61%), and five PDs (12%) were noted in total of 41 patients with PTCs (33 chemotherapy naïve, eight with prior chemotherapy). Each bar represents an individual patient. Chemotherapy naïve (blue) or prior chemotherapy (gold) status is noted by different color of the bar. (B) Kaplan-Meier analysis of progression-free survival (PFS) among all PTC patients (n = 41) who received at least one dose of sorafenib. For PTC chemotherapy-naïve patients (n = 33), median PFS is 16 months with 95% CI of 8 to 27.5. For PTC patients with prior chemotherapy (n = 8), median PFS is 10 months with 95% CI of 4 to 28. Using log-rank test to compare the curves for PFS, no statistically significant difference (P = .8627) was found in PFS between PTC groups. (C) Kaplan-Meier analysis of overall survival (OS). OS among all PTC patients (n = 41) who received at least one dose of sorafenib. For PTC chemotherapy-naïve patients (n = 33), median OS is 23 months with 95% CI of 18 to 34). For PTC patients with prior chemotherapy, median OS is 37.5 months with 95% CI of 4 to 42.5. Using log-rank test to compare the curves for OS, no statistically significant difference (P = .4787) was found in OS between PTC groups.