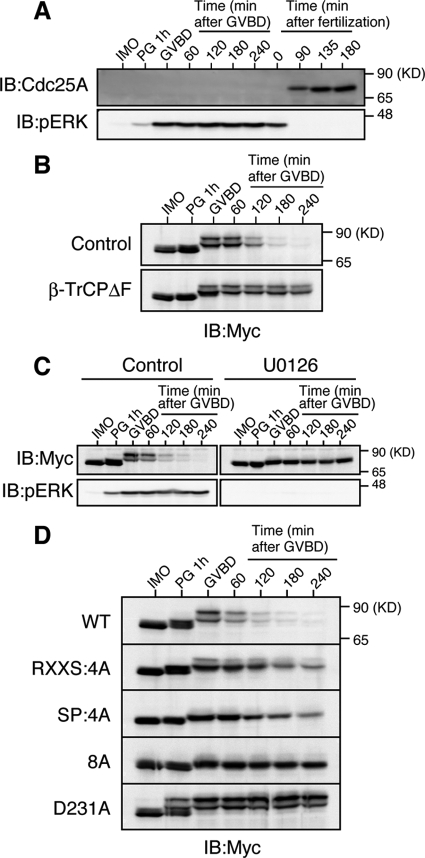
Figure 5.
ERK pathway-dependent degradation of Xe-Cdc25A during oocyte maturation. (A) Immature oocytes (IMO) were treated with progesterone (PG) to induce maturation, whereas ovulated eggs were fertilized in vitro. Maturing oocytes and fertilized eggs were collected at the indicated times, and analyzed for endogenous Xe-Cdc25A and phospho-ERK by immunoblotting. GVBD denotes germinal vesicle breakdown. (B) Immature oocytes were coinjected with 2 ng of Myc-Xe-Cdc25A mRNA and 20 ng of either GST mRNA (Control) or β-TrCPΔF mRNA, cultured overnight, treated with progesterone, and analyzed for Myc-Xe-Cdc25A by immunoblotting. (C) Immature oocytes were injected with 2 ng of Myc-Xe-Cdc25A mRNA, cultured overnight, pretreated with dimethyl sulfoxide (Control) or 100 μM U0126 for 1 h, treated with progesterone, and analyzed for Myc-Xe-Cdc25A and phospho-ERK by immunoblotting. (D) Immature oocytes were injected with 2 ng of mRNA encoding the indicated Myc-Xe-Cdc25A constructs, cultured overnight, treated with progesterone, and analyzed for Myc-Xe-Cdc25A constructs by immunoblotting. In B–D, all the Myc-Xe-Cdc25A constructs were, in fact, phosphatase-dead C428S forms to avoid premature maturation of the oocytes. Four, three, four, and five independent experiments were performed for A, B, C, and D, respectively, and, for each, a typical result is shown.