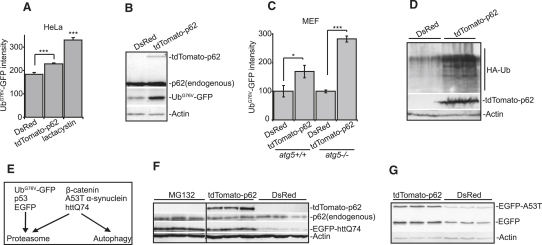
Figure 3.
Overexpression of p62 Inhibits Degradation of Proteasomal Substrates
(A) p62 overexpression increases levels of UbG76V-GFP. HeLa cells were cotransfected with UbG76V-GFP and either DsRed or tdTomato-p62 (1:3 ratio) and incubated for 48 hr. A separate set of UbG76V-GFP/DsRed-transfected cells were treated with 10 μM lactacystin 16 hr prior to analysis as a positive control. The GFP fluorescence intensity of double-positive green/red cells was quantified by FACS.
(B) Levels of UbG76V-GFP are increased in the presence of overexpressed p62. SK-N-SH cells were transfected as in (A). After 48 hr, cells were harvested and immunoblotted. The levels of exogenous p62 appear to be low because they are measured in the whole cell population from a transient transfection experiment, where only a proportion of cells express the transgene.
(C) p62 overexpression causes accumulation of UbG76V-GFP in wild-type and autophagy-deficient cells. atg5+/+ and atg5−/− MEFs were transfected as in (A), and the GFP fluorescence intensity of double-positive green/red cells was analyzed by FACS. We normalized the levels in both atg5+/+ and atg5−/− MEFs in control conditions to 100% to facilitate comparisons; however, the levels of UbG76V-GFP are higher in the autophagy-deficient MEFs.
(D) Overexpression of p62 leads to accumulation of ubiquitinated proteins. SK-N-SH cells were transfected with HA-Ub and either DsRed or tdTomato-p62 (1:1 ratio), incubated for 48 hr, and analyzed for levels of HA-Ub-labeled proteins by immunoblotting.
(E) Schematic diagram of degradation pathways for proteins used in this study.
(F) p62 increases levels of soluble polyQ. HeLa cells were transfected with EGFP-httQ74 and either DsRed or tdTomato-p62 (1:3 ratio) and were incubated for 48 hr. A separate set of EGFP-httQ74/DsRed-transfected cells was treated with the proteasomal inhibitor MG132, as a positive control.
(G) p62 increases levels of soluble mutant α-synuclein (EGFP-A53T) and EGFP. HeLa cells were transfected with EGFP-A53T, EGFP, and either DsRed or tdTomato-p62 (1:1:3 ratio) and were incubated for 48 hr prior to immunoblotting.
For all of the graphs, data are shown as means ± SE for three separate experiments performed in triplicate. ∗p < 0.05, ∗∗∗p < 0.005, t test; all other comparisons are not significant (n.s.).