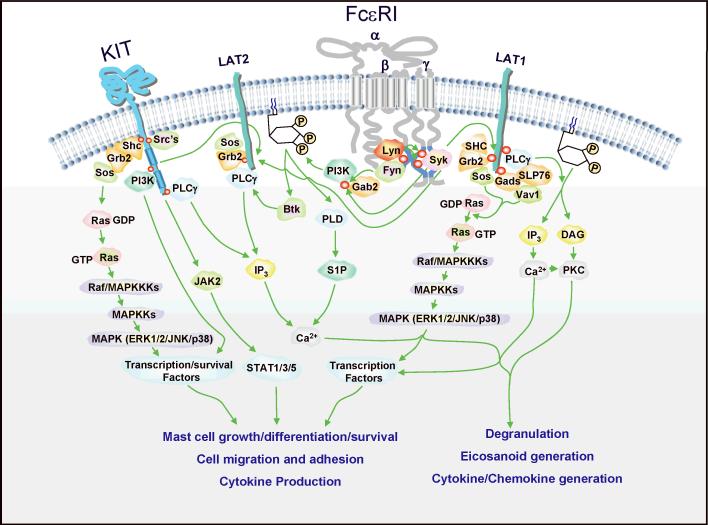
Fig. 1. Signaling pathways leading from activated KIT and aggregated FcεRI to mast cell responses.
Antigen-induced aggregation of IgE-occupied FcεRI induces activation of the Src family tyrosine kinase, Lyn, whereas SCF-induced KIT dimerization induces activation of its intrinsic KIT kinase activation. Phosphorylation of tyrosine residues within the receptor chains thus allows recruitment of SH2 domain-containing signaling molecules. In the case of FcεRI, Syk is recruited via ITAMs contained in the γ chain-cytoplasmic domains. Resulting activation of Syk, following its phosphorylation, leads to consequential phosphorylation of the transmembrane adapter molecules LAT1 and LAT2 (NTAL/LAB). Upon phosphorylation, these proteins serve as scaffolds for multimolecular signaling complexes comprising various cytosolic adaptor molecules such as Gads, Grb2, SLP76, and SHC, GTP exchangers including Sos and Vav1 and the signaling enzymes PLCγ1 and PLCγ2. PLCγ catalyzes the hydrolysis of PtdIns2 to yield diacylglycerol (DAG) and IP3, which, respectively, result in the activation of PKC and the liberation of intracellular calcium. Following depletion of the intracellular calcium stores, the calcium signal is maintained by store operated calcium entry (not depicted). These signals lead to mast cell degranulation and eicosanoid generation and also contribute to activation of transcription factors required for cytokine and chemokine production. In parallel to this pathway, PI3K is activated following binding to Gab2 upon the phosphorylation of this cytosolic adapter molecule by Fyn and/or Syk, phosphorylation of the p85α adapter subunit of PI3K, and activation of the catalytic subunit by small GTP-binding proteins. In the case of KIT, the p85α subunit directly binds to the phosphorylated molecule. The subsequent formation of membrane associated PtdInsP3 results in the recruitment of PH domain-containing signaling molecules such as Btk, PLD, and potentially others. PI3K-regulated pathways serve to enhance/maintain LAT/PLCγ1-regulated degranulation and, as depicted for KIT, regulate mast cell growth, differentiation, survival, migration, adhesion, and cytokine production. KIT- and FcεRI-mediated activation of the Ras-Raf-MAPK pathway following Sos- and Vav-regulated GDP-GTP exchange of Ras also contributes to these processes. The MAPK ERK1/2 also regulates PLA2 activation, which leads to the liberation of arachidonic acid for the generation of eicosanoids (not depicted). The role of LAT2 in mast cell activation is still enigmatic; however, it has been proposed to both upregulate and downregulate antigen-mediated responses. It does appear to be required for the ability of KIT to enhance FcεRI-dependent degranulation. Due to the complexity of the signaling cascades depicted, some of the intermediary steps involved in these processes could not be illustrated in this figure. For further details, readers are referred to other recent review articles (6,7,11).