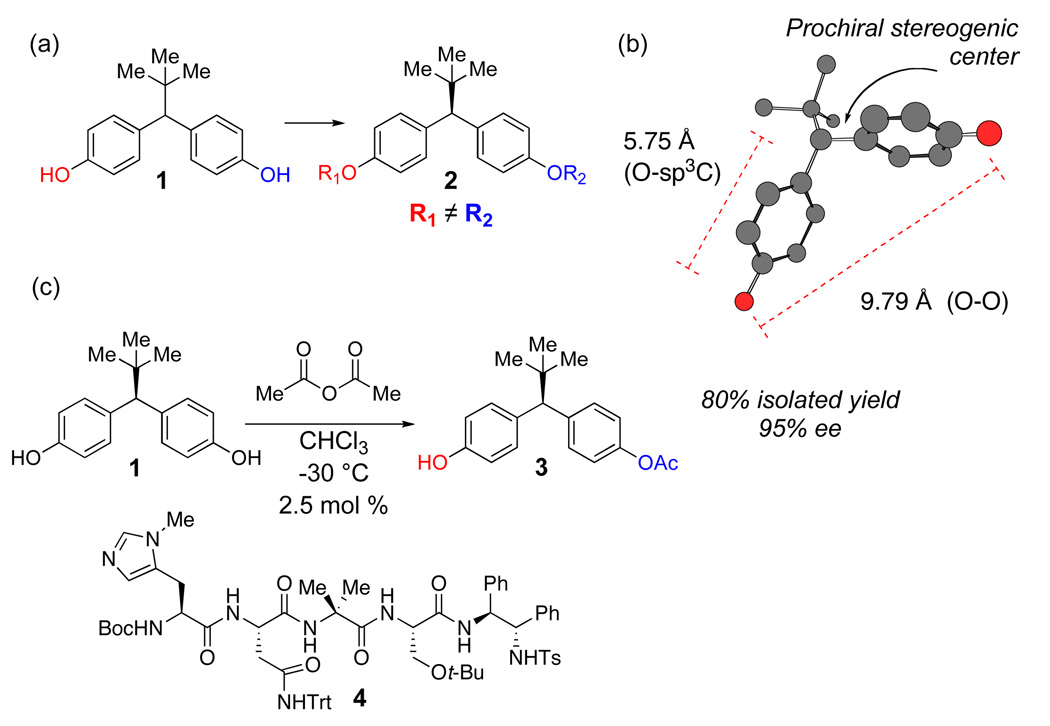
Figure 1.
(a) Compound 1 and its desymmetrized analog 2. (b) Substrate metrics for bis(phenol) 1, defining a 5.75 Å distance between desired site of functionalization and prochiral stereogenic center (MM calculations). 9.79 Å span the enantiotopic hydroxyl groups. (c) Catalyst 4 effects enantioselective desymmetrization with high enantiomeric excess.