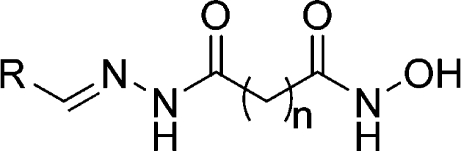
Table 3. Inhibition of P. falciparum Growth and pfHDAC-1 Activity by Cherry-Picked Hits from the HDAC-Biased Chemical Library.
| compd | R | n | pfHDAC-1, IC50 (nM) | fold change in MM.1S histone acetylationa | P. falciparum3D7, IC50 (nM) |
|---|---|---|---|---|---|
| 8 | 3-hydroxyphenyl | 5 | 59 ± 6 | 1.2 | 99 ± 12 |
| 9 | 2,5-dihydroxyphenyl | 6 | 110 ± 13 | 1.1 | 142 ± 28 |
| 10 | 6-bromobenzo[d][1,3]dioxol-5-yl | 5 | 20 ± 2 | 1.0 | 59 ± 15 |
| 11 | 2,4,6-trishydroxyphenyl | 6 | 43 ± 5 | 1.1 | 50 ± 6 |
| 12 | 2-bromo-5-methoxyphenyl | 5 | 22 ± 2 | 1.1 | >500 |
| 13 | 4-(dimethylamino)-2-hydroxyphenyl | 5 | 37 ± 4 | 1.2 | 24 ± 2 |
| 14 | 2-hydroxynaphthalen-1-yl | 5 | 41 ± 4 | 1.0 | 20 ± 2 |
| 15 | 2-bromo-5-hydroxyphenyl | 5 | 49 ± 5 | 1.0 | 35 ± 4 |
| 16 | 2-bromophenyl | 5 | 37 ± 4 | 1.1 | 15 ± 2 |
| 17 | 2-bromo-4-hydroxy-5-methoxyphenyl | 5 | 59 ± 7 | 1.0 | 47 ± 7 |
| 18 | 2-chlorophenyl | 5 | 90 ± 10 | 0.9 | 288 ± 20 |
| 19 | 4-(1H-imidazol-1-yl)phenyl | 5 | 15 ± 2 | 1.0 | 57 ± 11 |
| 20 | 2-bromopyridin-3-yl | 5 | 36 ± 6 | 0.9 | 22 ± 5 |
| 21 | 2-bromo-4-hydroxyphenyl | 4 | 106 ± 11 | 1.1 | 53 ± 13 |
| 22 | 2-bromo-5-hydroxyphenyl | 4 | 89 ± 9 | 1.1 | 30 ± 7 |
| 23 | 2-bromo-4-hydroxy-5-methoxyphenyl | 4 | 59 ± 5 | 1.2 | 498 ± 168 |
| 24 | 4-boronophenyl | 4 | 45 ± 5 | 1.0 | 68 ± 8 |
The fold change in MM.1S histone acetylation was measured at a compound concentration of 0.2 μM.