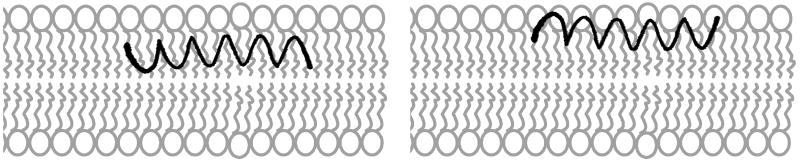
Figure 12.
Models of SP-B59-80 interactions with the two lipid environments studied assuming a helical peptide conformation. (Left) Based on 2H NMR data, SP-B59-80 deeply penetrates into 4:1 DPPC:POPG lipid bilayers. (Right) In contrast, in 3:1 POPC:POPG MLVs lipids, a more peripheral interaction of SP-B59-80 with the lipid headgroup region is proposed. A transmembrane orientation of the peptide is unlikely as it would place four polar residues in the hydrophobic interior.