Abstract
The hallmark of effective establishment of immune memory is the long-term memory cell that persists in the absence of antigen. To explore its characteristics, we investigated the differences between a resolved successful immune response, such as after influenza (flu) vaccination, and the state of chronic infection with persistent antigen, such as with cytomegalovirus (CMV), Epstein–Barr virus (EBV) or human immunodeficiency virus (HIV), which leads to defective T-cell memory. Immunophenotypic analyses using multi-parameter flow cytometry and tetramer technology identified a unique pattern of CD26high expression among influenza-specific CD8+ T cells, but not among CD8+ T cells specific for CMV, EBV (three different epitopes) or HIV. The median percentage of CD8+ T cells expressing CD26 was 95·5% for influenza, but for cells specific for CMV, EBV and HIV it was 10·5%, 12%–19%, and 13·2%, respectively. These findings suggest that expression of CD26high may be a characteristic of a memory cell. CD26high expression correlates with expression of CD127, a marker of memory cells. Furthermore, CD26high cells can produce interleukin-2. These findings offer insight into the dynamics of T-cell differentiation, and they may offer a specific marker of a successfully developed memory CD8+ T cell, that of CD26high. This marker has the potential to be useful in studies of immune responses to infectious agents, and to new vaccine candidates.
Keywords: CD26, CD8+ T cells, T-cell markers, virus-specific
Introduction
Post-thymic T-cell activation and differentiation is a complex and multifactorial process.1–4 It is generally believed that naïve cells, upon primary antigenic stimulation, give rise to effector cells; after initial expansion, the effector pool contracts and most cells succumb to apoptosis while a minority becomes long-lived memory cells.1–4 Several markers can differentiate between naïve and memory cells; however, only a few molecules can differentiate between memory cells and activated effector cells (reviewed in ref. 1). The pathways that influence the generation of effector and memory T cells are still largely undeciphered and the lineage relationships are controversial. The question, however, is of central importance in the search for insight into lymphocyte memory development and for suitable markers of an effective immune response for vaccine studies.
Examination of antigen-specific lymphocyte responses at different stages of particular infections offers in vivo‘snapshots’ of various phases of lymphocyte differentiation. Persistent viral infections in humans, such as cytomegalovirus (CMV), are marked by the presence in peripheral blood of effector phenotypes with varying degrees of late and terminally differentiated cells, as we and others have shown.5–7 Markers such as the killer cell lectin-like receptor G1 (KLRG1) and CD57, characteristic of antigen-experienced cells with effector function but poor replicative capacity, are expressed at a greater frequency on cells specific for agents of persistent infections at the chronic stage, but CD27 and CD28 are typically not expressed.5–7 In contrast, immune responses to infections that are acute and subsequently cleared with no persistence of antigen (such as influenza) are characterized, long-term, by the presence of true resting memory cells.6 Recent observations suggest that memory CD8+ T cells that can persist long-term in the absence of antigen have advantages over CD8+ T cells specific for persistent infections. A recently identified characteristic of memory cells is the expression of high levels of CD127, the α-chain of the interleukin-7 (IL-7) receptor.8 Such memory T-cell markers, in particular those associated with long-term protective immunity, need to be further explored. We approached this issue by performing a detailed immunophenotypic analysis of antigen-specific cells that are present in the absence of antigen, such as after successful influenza vaccination, and compared them with those of chronic infections with persistence of antigen and antigen-dependent memory development,8 such as CMV, Epstein–Barr virus (EBV) or human immunodeficiency virus (HIV). Our analyses yielded a unique CD26 expression pattern in influenza-specific CD8+ T cells, different from that observed in CD8+ T cells specific for CMV, EBV or HIV. CD26 is a marker expressed on activated lymphocytes that acts as a costimulatory molecule;9,10 mainly expressed on CD4+ T cells, it is thought to be a marker of T helper type 1 cells11 and its expression confers higher antigen sensitivity upon restimulation.12 It therefore has characteristics that are consistent with a memory cell marker; examining its patterns of expression on CD8+ T cells specific for agents of acute resolved and persistent infections might offer insights into T-cell memory development.
Materials and methods
Study subjects and samples
Blood samples were obtained from 23 healthy volunteers known to be seropositive for CMV and/or EBV (13 and 10, respectively) using commercially available serological tests; they were either human leucocyte antigen (HLA) B8+ (n = 11) or A2+ (n = 12). Ten of the donors had received previous influenza immunizations and were HLA A2+, with normal CD4 : CD8 ratios. The donors’ age range was 27–40 years; 12 were men. Subjects were recruited at the Emory University Vaccine Center; blood samples were provided in heparin or sodium citrate. We also used blood samples from four HIV-infected patients who participated in an HIV vaccine study; they were free of active concurrent infections at the time of testing, and their HIV infection was stable with low log10 viral loads (2·6–3·5 cps/ml) on antiretroviral therapy; these were HLA A2+. All subjects were recruited at the Emory University Vaccine Center and gave informed consent for the study.
Major histocompatibility complex class 1-peptides
Soluble major histocompatibility complex (MHC) class 1 peptide tetramers carrying cytotoxic T-lymphocyte (CTL) epitopes of CMV and influenza virus proteins were produced as described elsewhere.13,14 The HLA restriction, peptide sequences, virus and name of the gene products of derived CTL epitopes are presented in Table 1. The tetramers were prepared with streptavidin coupled to allophycocyanin (Molecular Probes, Eugene, OR). The epitopes selected are major dominant epitopes for CMV, EBV and influenza virus. For HIV we examined gag epitopes.
Table 1.
Major histocompatibility complex (MHC) class I/peptide tetramers and the respective human leucocyte antigen (HLA) restriction and cytotoxic T-lymphocyte (CTL) epitopes used in this study. The epitopes belong to antigens of the viral agents shown
| MHC/peptide tetramers | HLA restriction | CTL epitope | Viral agent |
|---|---|---|---|
| A2/CMV.NLV | A*0201 | NLVPMVATV | Cytomegalovirus (CMV) |
| A2/FLU.GIL | A*0201 | GILGFVFTL | Influenza virus (flu) |
| B8/EBV. FLR | B*0801 | FLRGRAYGL | Epstein–Barr virus (EBV) |
| B8/EBV.RAK | B*0801 | RAKFKQLL | Epstein–Barr virus (EBV) |
| A2/EBV.GLC | A*0201 | GLCTLVAML | Epstein–Barr virus (EBV) |
| A2/HIV.GAG | A*0201 | SLYNTVATL | Human immunodeficiency virus type 1 (HIV) |
Immunophenotyping and flow cytometry
A whole-blood flow cytometry technique was employed as described previously.5 Cells were stained with monoclonal antibodies (mAbs) labeled with fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein or allophycocyanin or tetramers. The whole-blood samples (200 μl) were stained at room temperature for 20 min; red blood cells were lysed in fluorescence-activated cell sorter (FACS) lysing solution (BD Biosciences, San Jose, CA) for 10 min in the dark, washed twice in FACS buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0·1% NaN3) and fixed in 300 μl 1% paraformaldehyde. The following mAbs (Beckman Coulter, Fullerton, CA) were used: anti-CD3 (clone UCHT1), anti-CD28 (clone CD28.2), anti-CD57 (clone NC1) and anti-CD127 (clone R34.34), CD27 (clone1A4CD27), CD11a (clone 25.3), CD45RA (clone ALB11), CD62L (clone DREG56). The following mAbs from BD Pharmingen (San Jose, CA) were used: anti-CD4 (clone SK3), anti-CD8 (clone SK1), anti-CD26 (clone L272), CCR5 (clone 2D7) and perforin (clone δG9). The CCR7 (clone 150503) was from R&D Systems (Minneapolis, MN) and the Granzyme-B (clone GB12) was from Caltag (Burlington, CA). The granzyme B and perforin were stained intracellularly for 20 min after permeabilization with FACS-Perm (BD Biosciences) for 10 min at room temperature, washed with FACS buffer and fixed with paraformaldehyde. Interferon-γ (IFN-γ; clone B27) and IL-2 (clone MQ1-17H12) were both from BD Pharmingen. The Alexa-KLRG1 (13A2) was kindly provided by Dr Pircher, University of Freiburg, Germany.
Peripheral blood mononuclear cell isolation and intracellular cytokine staining
Peripheral blood mononuclear cells (PBMC) were isolated over lymphocyte separation medium (Cellgro, Herndon, VA); 1 × 106 PBMC were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) and calcium ionophore A23187 (1 μg/ml) (Sigma-Aldrich, St Louis, MO) in 200 μl RPMI-1640/10% fetal bovine serum medium containing Golgi Plug (BD Pharmingen) for 6 hr. A negative control (non-stimulated) was included to control for spontaneous production of IFN-γ or IL-2. The PBMC were then surface-stained for 20 min at room temperature and lysed with 2 ml FACS Lyse (BD Biosciences) for 10 min at room temperature. The cells were washed twice with FACS-buffer (phosphate-buffered saline with 0·5% bovine serum albumin and 0·1% NaN3), permeabilized for 10 min at room temperature with 500 μl of FACS-perm (BD Biosciences), washed with FACS buffer, stained with IFN-γ (clone B27) or IL-2 (clone MQ1-17H12) for 20 min at room temperature, washed again, and fixed with 1% paraformaldehyde before acquisition on a FACS Calibur (BD Biosciences). FACS data were analysed with FlowJo software (Treestar, Carlos, CA).
Proliferation in vitro
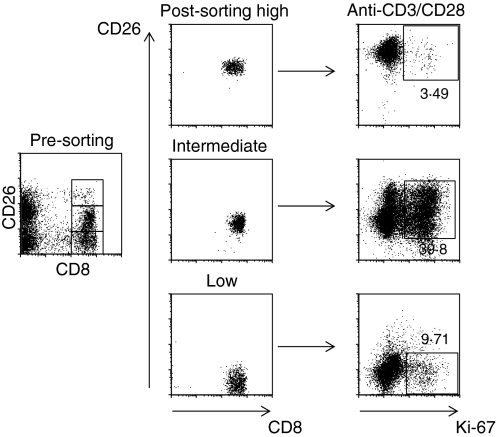
PBMC were sorted into CD8+ CD26high CD8+ CD26intermediate and CD8+ CD26low populations using a FACSAria (BD Biosciences). The purity of these cells was > 95%. These cells were stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) for 5 days at 37° before staining for intracellular Ki-67 (a marker of cell proliferation). Briefly, cells were washed, stained with surface antibodies, permeabilized for 10 min at room temperature with 500 μl FACS-perm (BD Biosciences), and then washed with FACS buffer before staining intracellularly with Ki-67 (clone B56) for 25 min at room temperature. The cells were washed again and fixed with 1% paraformaldehyde before acquisition on a FACS Calibur. Each experiment was repeated three times.
Results
Expression of CD26 by the bulk population of CD8+ T lymphocytes: three subsets and their characterization
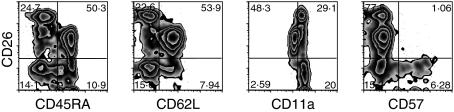
CD26 staining on CD3+ CD8+ T cells identified three subsets of cells: CD26low, CD26intermediate and CD26high (Fig. 1). The CD26high subset was present in variable proportions in the subjects studied, ranging from 1·8% to 22% (median, 7·5%) of all CD8+ T cells. We further characterized these subsets by performing immunophenotypic analyses on CD3+ CD8+ T cells with a panel of naïve and antigen-experienced cell markers. Costaining with CD45RA, traditionally thought of as a marker of naïve T cells, identified all cells within the CD26high and some of the cells in the CD26low subset as CD45RA− but the majority of the cells in the CD26intermediate subset as CD45RA+ (Fig. 1). A similar pattern was observed with CD62L, another marker mainly of naïve cells (Fig. 1). Conversely, staining with CD11a, a marker of antigen-experienced T cells, showed that CD26high and CD26low cells expressed high levels of CD11a, whereas the majority of CD26intermediate cells expressed low levels of CD11a (Fig. 1). Taken together, these results indicate that expression of CD26high identifies a population of antigen-experienced cells, whereas expression of CD26intermediate identifies a mostly, but not exclusively, naïve population of cells. CD26low identifies mostly antigen-experienced cells, including some CD57+ terminal effectors (Fig. 1).
Figure 1.
Characterization of the CD26 subsets of human CD8+ T lymphocytes in a representative individual. The majority of CD26high CD8+ T cells are CD45RA−, CD62L− and CD11a+. The same is true for the majority of CD26low CD8+ T cells, while the reverse is true for the majority of CD26intermediate CD8+ T cells. CD57+ cells (a marker of replicative senescence) are only found in the CD26low subset. Gated on unstimulated CD8+ CD3+ T cells.
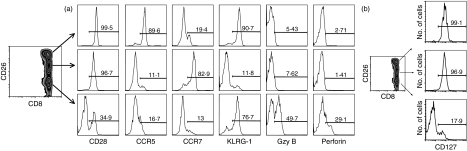
To further define the functional properties of the CD26 subsets of CD8+ T cells, we used four-colour flow cytometry for coexpression of a number of key markers associated with cytotoxicity and effector and memory function. Given that our initial characterization indicated that CD26intermediate cells were mostly naïve cells, we wanted to further distinguish the function of CD26high and CD26low cells, which both bore activated cell phenotypes. As shown in Fig. 2, the differences between the two subsets were in expression of CD127, CCR5 and CD28 (with the majority of CD26high cells, but not of CD26low cells, expressing these markers) and in expression of perforin and granzyme B (where, in contrast, only a very small fraction of CD26high cells were expressing, but one-third to one-half of CD26low cells did). Taken together, these results portray an antigen-experienced, activated but non-effector phenotype for CD26high cells, whereas CD26low cells seem to contain heterogeneous phenotypes, including effectors and some CD45RA+‘terminal’ effectors (Figs 1, 2). Both CD26high and CD26lowCD8+ T-cell subsets expressed KLRG-1 (Fig. 2).
Figure 2.
Detailed immunophenotypic characterization of the CD26 subsets of CD8+ T lymphocytes. Expression of CD28, CCR5 CCR7, KLRG-1, granzyme B (Gzy B), perforin (a) and CD127 (b) in the CD26 subsets of human CD8+ T lymphocytes. Whole blood from the donors was stained with monoclonal antibodies specific for each marker shown. Histograms on the right display expression of the respective marker gated on the region of each CD26 subset on the left. Panels from representative donors are shown.
Distinct patterns of CD26 expression on CD8+ T cells specific for different viral agents: chronic, active and cleared infection
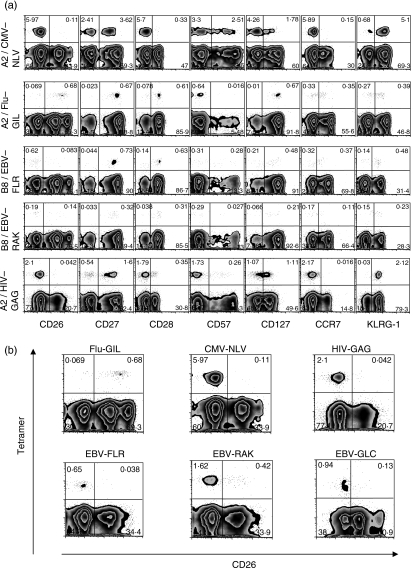
The CD8+ T-cell responses specific to viruses causing persistent infection, such as CMV, EBV, hepatitis C virus and HIV, have been shown to be qualitatively different at the chronic stage depending on viral specificity.6,7 We undertook a comparison of CMV, EBV and HIV, all three chronic infections, with influenza, an infection that resolves without ongoing viral persistence. We characterized antigen-specific responses at the chronic stage of CMV, EBV and HIV and at the stage several months after influenza immunization. As we and others have previously described,5,6,15 CD8+ T lymphocytes specific for CMV epitopes are enriched in CCR7−, CD28−, KLRG1+ and CD57+ cells, characteristic of ‘effector’ progressing to ‘terminally differentiated’ types of lymphocyte. Some of them express the CD127 marker, previously shown to denote a cell destined to become a long-lived memory cell,16 but at a low intensity (Fig. 3a). In contrast, CD8+ T cells specific for influenza virus, in the absence of contemporaneous antigenic stimulation, have lower levels of expression of KLRG1, do not express CD57, and are enriched in CCR7+, CD28+ and CD127high cells (Fig. 3a).
Figure 3.
Characteristics of CD8+ T human lymphocytes specific for cytomegalovirus (CMV; top panel), influenza virus (Flu; second panel) Epstein–Barr virus (EBV; third and fourth panels) and human immunodeficiency virus (HIV; bottom panel). (a) Four representative individuals are presented. The majority of CMV-specific cells were CD26low, CD28−, CCR7− and enriched in CD27−, CD127−, KLRG1+ and CD57+ cells. Similar CD26 expression was obtained for the two EBV epitopes studied, as well as for HIV. Conversely, influenza-virus-specific cells were a majority of CD26+, CD27+, CD127+, CD57−, and enriched in CD28+ and KLRG1− cells. Gated on unstimulated CD8+ CD3+ T cells. (b) Focus on CD26 expression on different virus-specific CD8+ human T cells.
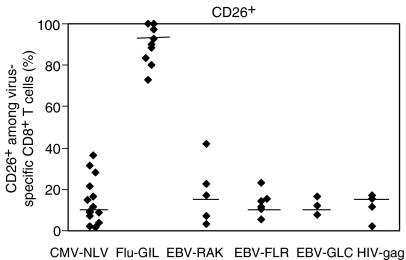
The use of CD26 offers a striking distinction of the responses to the two types of infection. The majority of CMV-specific CD8+ T lymphocytes are CD26low without any CD26high representation (Fig. 3a,b). Similar results were obtained for the EBV epitopes FLR and RAK, as well as for HIV (Fig. 3a,b). In contrast, the great majority of flu-specific cells are CD26high (Fig. 3a,b). The median percentage of CD8+ T cells expressing high or intermediate CD26 was 95·5% (range 80–100%) for influenza, but only 10·5% (range 1·8–36·7%) for CMV (P = 0·03), between 12·1% and 19·5% for each of the three EBV epitopes studied (RAK, FLR and GLC) (range 3·4–23%) and 13·2% for HIV (range 2–17%) (Fig. 4). Together, these findings show that influenza-specific cells, contrary to cells specific for CMV, EBV and HIV, express CD26 and CD127 but only a proportion of them expresses KLRG1 and CD57. In addition, there was much more marked representation of CD28+ cells among influenza virus-specific CD8+ T lymphocytes, compared with CMV-specific cells, as has been previously described.6,7 Interestingly, there were also differences in the responses to the different EBV epitopes, with a more marked CD28+ response to FLR (a latency-associated antigen) than to RAK (a lytic protein epitope). The influenza virus-specific phenotype would be most consistent with a ‘memory’ population, according to the schemes proposed by Sallusto and Lanzavecchia17 and an ‘early’ phenotype according to Appay et al.,7,18 whereas the CMV-specific responses would be ‘effector’ or ‘intermediate–late’ phenotypes, respectively.
Figure 4.
Percentages of CD26 expression among human CD8+ T lymphocytes specific for cytomegalovirus (CMV), Epstein–Barr virus (EBV), human immunodeficiency virus (HIV) and influenza virus (Flu). Each point within the particular virus group represents the value of one donor. Medians for each virus group have been drawn. All donors were analysed in the chronic (CMV, HIV and EBV) or post-acute (Flu) phase of the infection.
In vitro stimulation: proliferation, cytokine production and CD26 expression
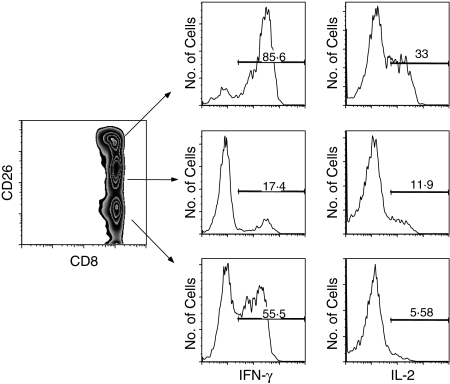
On stimulation of CD8+ T cells with PMA/ionomycin, IFN-γ was produced by both CD26high and CD26low cells, whereas IL-2 was produced mostly by CD26high cells (Fig. 5). Approximately 56% of CD26low cells produced IFN-γ but less than 6% produced IL-2 upon stimulation with PMA/ionomycin. In contrast, 86% of CD26high produced IFN-γ and 33% produced IL-2. To investigate the in vitro proliferative responses of CD3+ CD8+ T cells, we sorted the cells according to their level of CD26 expression and then stimulated them with anti-CD3 and anti-CD28 for 5 days. We observed that there was up-regulation of CD26 expression upon such stimulation within all CD26 subsets. The highest levels of proliferation, as measured by Ki-67 expression, were seen in the CD26intermediate compartment, where a substantial proportion of the cells had become CD26high. A representative experiment is shown in Fig. 6.
Figure 5.
Interferon-γ (IFN-γ; left panel) and interleukin-2 expression (IL-2; right panel) of the CD26 subsets of CD8+ human T cells upon stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin. The IFN-γ is produced by both CD26low and CD26high cells, whereas IL-2 is produced mostly by CD26high cells. Results from a representative experiment are shown.
Figure 6.
Proliferative capacity of the sorted CD26 subsets of CD8+ T cells upon stimulation with anti-CD3/CD28. The CD26intermediate cells and the CD26high derived from CD26intermediate have the highest proliferative capacity. Results from one representative experiment are shown.
Discussion
This study identified a unique pattern of CD26 expression on antigen-specific CD8+ T cells that recognize epitopes of a viral agent that causes acute resolved infection (influenza). This pattern is not observed among CD8+ T cells specific for agents causing chronic infection (CMV, EBV, HIV). Antigen-specific cells against these viral agents in the different disease stages studied represent functionally distinct phases of T-cell development. Long-term T-cell memory is regularly associated with strong initial antigenic stimulation that leads to antigen clearance.19 Infections with a high load of persisting antigen are characterized by sustained high levels of effector cells with poor memory formation and eventual exhaustion.19 It has been argued that progressive memory CD8 T-cell differentiation occurs in the absence of antigenic persistence and that CD127 is a marker of long-lived memory cells.16,20 In contrast, in states of antigen persistence CD8 T-cell differentiation may be defective,8 even though this view may have been challenged by recent findings that indicate that the appropriate signal can restore the proliferation of CD28− CD45RA+ CMV-specific CD8+ T cells, which were previously thought of as ‘terminally differentiated’, suggesting a greater functional plasticity of subpopulations of memory T cells than currently understood.21
Our findings indicate that the influenza-specific CD8 ‘memory’ T cells express CD26 in addition to CD127. Similar results were obtained for responses to vaccinia virus in two donors vaccinated with the vaccinia virus (data not shown). In contrast, CMV-specific and EBV-specific cells do not express CD26. High expression of CD26 among CD8+ T cells may be a marker of effective long-term memory T-cell formation. The lack of expression of CD26 on CD8+ T cells specific for CMV, EBV or HIV could be explained by either depletion of this subset or incomplete or arrested differentiation as a result of the action of regulatory cells, the dysfunction of antigen-presenting cells or other mechanisms.
This finding has implications for HIV immune pathogenesis and control: a deficient expression of CD26high on CD8+ T cells of HIV-infected patients has been previously described.22,23 HIV-specific CD8+ T-cell responses show features of impaired maturation in chronically infected patients,8,24 but are more efficiently maintained if antiretroviral therapy is initiated during the early phase of infection.25 These observations suggest the need to study CD26 expression in HIV-infected patients who are treated very early in their infection, or in long-term non-progressors. Such studies would further our understanding of the role of this marker in a chronic infection when its course is modified either naturally or by intervention.20 This would have important implications in the search for appropriate markers to assess immune responses to HIV candidate vaccines.
Moreover, our observation offers some additional insights into the more fundamental question of T-cell differentiation. CD26 seems to be expressed in intermediate intensity in the ‘naïve’ stage but to be either up-regulated or down-regulated in the antigen-experienced stages. The functional data on cytokine production and proliferative capacity concur with CD26high cells being a ‘memory’ population (that can produce IL-2), and CD26low cells being an ‘effector’ population (IFN-γ producers). However, CD26high‘memory’ cells also express KLRG1 and have reduced proliferative capacity, which may have implications for their function in long-term immunological protection.26 Of interest, cells of the CD26intermediate subset that up-regulate their CD26 expression upon in vitro stimulation retain their capacity for proliferation and may be more important in the ‘recall’ phase in vivo. Our data do not provide an explanation for the mechanisms that permit a subset of antigen-experienced cells to up-regulate the expression of CD26, whereas the majority of cells lose expression of this marker. Expression of CD26 could be dependent on the amount of antigen encountered by the T-cell receptor. The study by Kaech et al. demonstrated that IL-7Ra (CD127) was expressed on a minority of effector CD8+ T cells at the peak of the lymphocytic choriomeningitis virus response, suggesting that the commitment to memory is made at the peak of the infection and not during the contraction phase.16 In addition, all IL-7Ra(CD127)+ cells expressed granzyme B, indicating that memory cells do pass through an effector stage.27 Our experiments show that CD26high cells do not express granzyme B or perforin, which might argue for distinctly developed effector and memory T-cell stages or it might simply reflect differences in the experimental systems used. It will be important to study the signals and mechanisms involved in selective up-regulation of CD26 in a subset of cells and the exact functional significance of such up-regulation. Our preliminary observations suggest that CD26 might be a marker of memory cells; contrary to CD127, also a memory marker, CD26high is not expressed at the naïve stage.
Taken together, our results show different CD26 expression patterns on virus-specific cells during viral persistence and following a resolved immune response. The phenotypic characteristics of the CD26high subset and its correlation with CD127 expression suggest that it may be a memory T-cell subset that develops following a resolved acute infection. CD26 may prove to be a valuable tool in further studies of antigen-specific CD8 T-lymphocyte development, immunopathogenesis of HIV infection and vaccine responses.
Acknowledgments
This work was supported by CFAR grant #5P30A1050409 to the Immunology Core Laboratory, Emory Vaccine Center. We would also like to acknowledge the support from Cancer Research Institute Investigator Award, Woodruff Health Sciences Fund, the Yerkes Research Center Base Grant RR-00165 and the Public Health Service [K08 AI072191 (HR), AI070101 (AG)]. The authors wish to thank Dr H. Pircher for kindly providing the antibody used to identify KLRG1 in this study, Dr J. Altman for the tetramers and Ms P. Bansil for help with statistical analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–43. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 2.Chandok MR, Farber DL. Signaling control of memory T cell generation and function. Semin Immunol. 2004;16:285–93. doi: 10.1016/j.smim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Esser MT, Marchese RD, Kierstead LS, et al. Memory T cells and vaccines. Vaccine. 2003;21:419–30. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 4.Falcioni F, Shah H, Vidovic D, et al. Influence of CD26 and integrins on the antigen sensitivity of human memory T cells. Hum Immunol. 1996;50:79–90. doi: 10.1016/0198-8859(96)00121-8. [DOI] [PubMed] [Google Scholar]
- 5.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–94. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 6.Tussey LG, Sadasivan NU, Bachinsky M, Edwards B, Bakari J. Antigen burden is a major determinant of HIV-specific CD8+ T cell maturation state: potential implications for therapeutic immunization. J Infect Dis. 2003;187:364–74. doi: 10.1086/367707. [DOI] [PubMed] [Google Scholar]
- 7.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafler DA, Chofflon M, Benjamin D, Dang NH, Breitmeyer J. T cell activation and CD3 phosphorylation correlates with Ta1 (CDw26) expression. J Immunol. 1989;142:2590–6. [PubMed] [Google Scholar]
- 10.Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 11.De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–75. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 12.Northrop JK, Shen H. CD8+ T-cell memory: only the good ones last. Curr Opin Immunol. 2004;16:451–5. doi: 10.1016/j.coi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+ CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–8. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- 14.Voehringer DC, Blaser P, Bawand DH, Raulet T, Hanke T, Pircher P. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–43. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 15.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–6. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech SM, Tan JT, Wherry EJ, Konieczy BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 18.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–12. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–32. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Brostrom C, Sonnerborg A, Lindback S, Gaines H. Low relative frequencies of CD26+ CD4+ cells in long-term nonprogressing HIV type-1 infected subjects. Clin Diagn Lab Immunol. 1998;5:662–6. doi: 10.1128/cdli.5.5.662-666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28−CD45RAHI) CD8+ T cells. Blood. 2007;110:4630–6. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 22.Vanham G, Kestens L, De Meester I, Vingerhoets J, Penne J, Vanhoof G, et al. Decreased expression of the memory marker CD26 on both CD4+ and CD8+ T lymphocytes of HIV-infected subjects. J Acquir Immune Defic Syndr. 1993;6:749–57. [PubMed] [Google Scholar]
- 23.Blazquez MV, Madueno JA, Gonzalez R, Jurado R, Bachovchin WW, Pena J. Selective decrease of CD26 expression in T cells from HIV-1 infected individuals. J Immunol. 1992;149:3073–7. [PubMed] [Google Scholar]
- 24.van Baarle D, Kostense S, van Oers M, Hamann D, Miedema F. Failing immune control as a result of impaired CD8+ T cell maturation: CD27 might provide a clue. Trends Immunol. 2002;23:586–91. doi: 10.1016/s1471-4906(02)02326-8. [DOI] [PubMed] [Google Scholar]
- 25.Alter G, Hatzakis G, Tsoukas CM, Pelley K, Rouleau D, LeBlanc R, et al. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting HAART I primary infection. J Immunol. 2003;171:477–88. doi: 10.4049/jimmunol.171.1.477. [DOI] [PubMed] [Google Scholar]
- 26.Joshi NS, Cui W, Chandele A, Lee HK, Urso D, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–25. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]