Figure 5. Induction of HSV-1-specific T cell response in CJ9-gD-immunized mice.
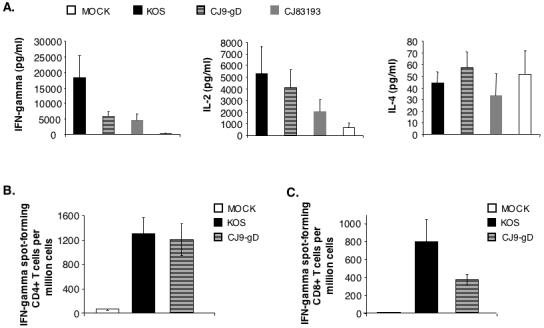
Cytokine assays (A). Female BALB/c mice were immunized with either mock-infected cell lysate, KOS, CJ9-gD or CJ83193 at 2 × 106 PFU per mouse. Splenocytes were isolated individually from mock-immunized (n = 4) and immunized mice (n = 4), and seeded in 24-well plates at 1.5 × 106 cells/well. Cells in duplicate were mock-stimulated or stimulated with UV-inactivated HSV-1 strain McKrae. Extracellular medium was collected at 72 h post-stimulation and levels of IFN-γ, IL-2, and IL-4 were determined. Cytokine production is presented as the mean concentration ± SEM in splenocytes isolated from 4 mice per group. IFN-γ ELISPOT assays (B and C). Splenocytes were prepared individually from mice (n = 4) either mock-immunized or immunized with KOS or CJ9-gD. For CD4+ T cell ELISPOT (B), CD4+ T cells were isolated from splenocytes using Dynal mouse CD-negative kit, and seeded in 96-well MultiScreen HTS™, IP sterile white filtration plates pre-coated with anti-mouse IFN-γ specific monoclonal antibody (AN18) at 5 × 104 and 1.5 × 105 cells/well. Cells in triplicate were stimulated with mock-infected or UV-inactivated HSV-1 strain McKrae infected- and mitomycin C-treated syngeneic CD11c+ BM-DCs. For detection of HSV-1-specific CD8+ T cells (C), splenocytes seeded in triplicate wells of 96-well filtration plates pre-coated with monoclonal antibody AN18 were stimulated with either mock-infected or HSV-1 strain McKrae-infected and mitomycin C-treated syngeneic CL7 cells. The IFN-γ spot-forming cells were detected as described in Materials and Methods. The HSV-1-specific IFN-γ spot-forming cells (SFC) are expressed as the mean ± SEM per million splenocytes from 4 mice per group.
