Abstract
Selective responses of retinal ganglion cells (RGCs) to the direction of motion have been recorded extracellularly from the rabbit and the mouse retina at eye opening. Recently, it has been shown that the development of this circuitry is light independent. Using whole-cell patch clamp recording, we report here that mouse early postnatal direction-selective ganglion cells (DSGCs) showed lower membrane excitability, lower reliability of synaptic transmission and much slower kinetics of light responses compared with adult DSGCs. However, the degree of direction selectivity of early postnatal DSGCs measured by the direction-selective index and the width of the directional tuning curve was almost identical to that of adult DSGCs. The DSGCs exhibited a clear selectivity for the direction of motion at the onset of light sensitivity. Furthermore, the degree of direction selectivity was not affected by rearing in complete darkness from birth to postnatal day 11 or 30. The formation of the retinal neurocircuitry for coding motion direction is completely independent of light.
A subgroup of retinal ganglion cells (RGCs) respond selectively to the direction of motion and are known as the direction-selective ganglion cells (DSGCs; Barlow & Hill, 1963; Barlow et al. 1964). Accumulating evidence suggests that cholinergic amacrine cells (or starburst amacrine cells, SACs) play an important role in producing direction-selective (DS) responses to moving targets. The terminal varicosities of SACs exhibit larger responses to centrifugal than to centripetal movement (Euler et al. 2002) through a cell autonomous mechanism (Hausselt et al. 2007) and/or through the interaction with overlapping SACs (Lee & Zhou, 2006). The SACs also contain the inhibitory neurotransmitter GABA (Brecha et al. 1988) and inhibit underlying DSGCs from the null side (Fried et al. 2002). Blockade of GABAA receptors abolishes direction selectivity in DSGCs (Caldwell & Daw, 1978).
Light is an important signal for development of the visual system. In the retina, deprivation of light in the early postnatal stage results in many changes, for example, bipolar cell differentiation appears to slow down from postnatal day 2 (P2) to P7 (Wu & Chiao, 2007); SACs undergo apoptosis (Zhang et al. 2005); GABA and Glutamate decarboxylase (GAD) expression is reduced (Lee et al. 2006); and the stratification of RGCs is delayed and synaptic input pattern to the RGCs is altered (Tian & Copenhagen, 2001, 2003; Xu & Tian, 2007). Given the above evidence, it is conceivable that light deprivation may lead to changes in direction selectivity. However, recent findings suggest that the formation of the DS circuitry is independent of light (Chan & Chiao, 2008; Elstrott et al. 2008) and of cholinergic activities (Elstrott et al. 2008).
The mechanisms regulating the formation of DS circuitry are still unknown (He et al. 2003; Zhou & Lee, 2008). The earliest detection of DS responses in the literature is at eye opening both in the rabbit and in the mouse (Bowe-Anders et al. 1975; Masland, 1977; Zhou & Lee, 2005; Chan & Chiao, 2008; Elstrott et al. 2008). A reduction in the time window will provide possible clues to aid understanding of the regulatory mechanisms responsible for the formation of this circuitry. Morphologically identifiable on–off DSGCs emerge as early as P0 in the rabbit (DeBoer & Vaney, 2005) and P3 in the mouse (Stacy & Wong, 2003; Diao et al. 2004; Coombs et al. 2007); their dendrites are already closely associated with processes of SACs, and the association increases with maturation, reaching a level comparable to the adult by P7 (Stacy & Wong, 2003). It is not clear whether the emergence of adult-like dendritic morphology is accompanied by formation of functional circuitry. It is also not clear how the physiological properties of early postnatal DSGCs differ from adult counterparts.
In this study, we attempted to answer two questions. First, what is the earliest emergence of retinal direction selectivity? Second, are physiological properties different between early postnatal and adult DSGCs? We found that retinal DSGCs exhibited clear selectivity for motion directions at the onset of light sensitivity, and the degree of DS was not significantly different between early postnatal and adult DSGCs, although many other properties, such as excitability of the cell membrane, and reproducibility and kinetics of the synaptic responses, were significantly different. Rearing animals in complete darkness did not change the ability of these cells to code motion direction. The answer to the first question advanced the emergence of retinal direction selectivity to the onset of light sensitivity and therefore reduced the window for exploring the mechanisms of wiring. The answer to the second question led to documentation of the physiological properties of early postnatal DSGCs. The maturation of cellular properties, such as membrane excitability and kinetics of synaptic responses, is not critical for the underlying mechanism of the computation of motion direction.
Methods
Whole-mount retina preparation
Mice of the C57BL/6N strain, aged P11–P13, P18 and adult (>P30), were used in this study. Some were reared in complete darkness from birth to P11 or to P30. Use and handling of animals were strictly in accordance with the guidelines at both the Institute of Biophysics and Institute of Neuroscience Chinese Academy of Sciences and with the Society for Neuroscience's policies on the use of animals and human subjects in neuroscience research. All experimental procedures have been previously described (Weng et al. 2005) and are briefly summarized here. Animals used to record light responses were dark adapted for at least 1 h, deeply anaesthetized with an i.p. injection of a mixture of ketamine (50 mg kg−1) and xylazine (10 mg kg−1) (Sigma, St Louis, MO), decapitated, and the eyes immediately enucleated under very dim red light. The retina was carefully dissected from the pigment epithelium in Ames’ medium equilibrated with 95% O2 and 5% CO2, and attached, ganglion cell side up, to a piece of black Millipore filter paper (AABP02500, Millipore, Billerica, MA) with a 2 mm diameter hole in the centre for adequate infrared illumination and visual stimulation. The whole-mount retinal preparation was then transferred into a recording chamber (0.5 ml in volume) on the fixed stage of an upright microscope (E600FN, Nikon) equipped with epifluorescence and a ×40 water-immersion objective (n.a. 0.8) configured for Differential interference contrast (DIC). The preparation was continuously superfused with oxygenated bicarbonate-buffered Ames’ medium at 32–35°C.
Patch clamp recording
Micropipettes were manufactured from thick-walled borosilicate filament glass tubing (1.5 mm outer and 0.86 mm inner diameter; Sutter Instruments Inc., San Rafael, CA, USA) using a Flaming–Brown P97 puller (Sutter Instruments Inc.). Under infrared illumination and visual control using a cooled CCD camera (Sensicam, Cooke, Auburn Hills, MI, USA), a pipette was advanced to the retina using an MP 285 micromanipulator (Sutter Instruments Inc.), and the inner limiting membrane was dissected to expose the somata of several RGCs. Retinal ganglion cells with an elliptic soma were targeted and their spike activities recorded in loose-patch mode with a pipette (2–4 MΩ) filled with Ames’ medium. Using a flashing spot and a moving bar, the on–off DSGC could be identified, its receptive field mapped and the preferred–null axis determined. We calculated a direction-selective index (DSI) by dividing the difference of the preferred and null responses by the sum of them. In the process of isolating the retina and exposing the RGCs, mechanical damage can easily depolarize DSGCs and result in weaker direction selectivity. Therefore, we only proceeded with DSGCs exhibiting clear direction selectivity, i.e. with a DSI greater than 0.3. For whole-cell voltage clamp recording, the extracellular pipette was replaced with a patch pipette with 4–7 MΩ resistance filled with intracellular solution (mm): caesium methanesulphonate, 120; CaCl2 0.5; EGTA 5; Hepes 10; ATP 4; GTP 0.5; and QX-314 5; adjusted to pH 7.2 with 1 m CsOH. The intracellular solution for whole-cell current clamp was (mm): potassium gluconate, 120; NaCl, 5; KCl, 10; MgCl2, 1; EGTA, 1; Hepes, 10; ATP, 2; and GTP, 0.5; adjusted to pH 7.2 with 1 m KOH. In some cases, 0.5% Neurobiotin (Molecular Probes, Eugene, OR, USA) and/or 0.1% Lucifer Yellow (Sigma) was added to reveal the dendritic morphology of the recorded cells. The whole-cell configuration was established when the seal resistance was > 1 GΩ. The liquid junction potential of 10 mV was always corrected. Data acquired from the Axopatch 200B amplifier were low-pass filtered at 2 kHz, digitized simultaneously with an A/D converter (Digidata 1320A, Axon Instruments) and stored on a personal computer. Offline data analysis was done using Clampfit (Axon Instruments) and Mini Analysis (Synaptosoft Inc., Leonia, NJ, USA), and plotted with OriginPro 7.0 (MicroCal Software Inc., Northampton, MA, USA).
Light stimulation
Stimuli were generated using a program written in VC++ and Directx8 SDK, displayed on a monitor (Sony E230) and focused onto the retina through a microscope condenser. Two types of light stimuli were generated: (1) a spot of 25–1000 μm in diameter, flashed for 0.5–10 s, was used to determine the size of the receptive field and response polarity; and (2) a rectangle of 100 μm × 500 μm moving parallel to its long axis in one of 12 directions with 30 deg intervals at about 750 μm s−1 over 1500 μm was used to determine the directionality. The elongated bar allowed a clear separation of leading edge (on) and trailing edge (off) responses.
Visualization of recorded cells
Retinae were fixed with 4% paraformaldehyde for 1 h and washed three times in 0.1 m phosphate buffer (pH 7.4). Neurobiotin injected into the DSGCs was visualized with streptavidin–fluorescein isothiocyanate (FITC) or streptavidin–Texas Red (Vector Laboratories, Burlingame, CA). Some DSGCs injected with Lucifer Yellow were incubated with rabbit anti-Lucifer Yellow antibodies (1:100, Molecular Probes) and 0.5% Triton X-100 for 2 days, and visualized with anti-rabbit FITC antibodies (1:100, Jackson Laboratory, Bar Harbor, Maine). Preparations were coverslipped with Vectorshield (Vector Laboratories), and sealed with nail polish. Images were collected using a Nikon Eclipse E800 microscope equipped with Plan Apo ×10, n.a. 0.45; ×20, n.a. 0.75; and ×40, n.a. 1.0 objectives and a CCD camera (Cascade, Photometrics, Tucson, AZ). The contrast and brightness of the images were adjusted using Photoshop 8.0 (Adobe).
Results
Identification of on–off DSGCs in P11 retina
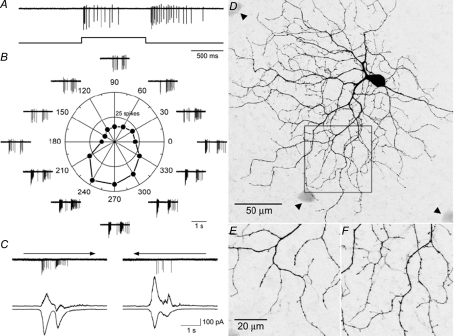
We detected on–off DS responses in the mouse retina as early as P11, 3 days before eye opening and as soon as the RGCs responded to our visual stimuli. These cells exhibited relatively transient responses at both the onset and termination of a stationary flashing spot (Fig. 1A), and clear directional responses to a rectangle moving in 12 directions (Fig. 1B). Whole-cell voltage clamp recordings showed that these cells received a larger excitatory input when the visual stimulus was moving in the preferred directions, and a larger inhibitory input when the same stimulus was moving in the opposite, null directions (Fig. 1C). Visualization of intracellularly infused Neurobiotin revealed a clearly bistratified dendritic morphology (Fig. 1D–F), observed in many studies of mouse RGCs (Sun et al. 2002; Badea & Nathans, 2004; Kong et al. 2005; Coombs et al. 2006) and identified as on–off DSGCs in adult mouse retina (Weng et al. 2005). Further analyses showed the preferred directions for the mouse DSGCs were pointed towards anterior, posterior, superior and inferior, very similar to what we found in adult mice (Supplementary Fig. 1), and consistent with the findings from the rabbit and the mouse (Oyster, 1968, Elstrott et al. 2008).
Figure 1. Direction-selective ganglion cells from P11 mouse retina.
A, response to a stationary spot flashing for 1000 ms, showing transient on- and off-responses. B, polar plot showing responses to a rectangle moving in 12 directions. Strong direction selectivity is apparent. C, synaptic inputs to the on–off DSGCs. The upper traces show spike responses to a stimulus moving in the preferred and null direction. Arrows indicate directions of movement. The lower traces are synaptic currents recorded when the membrane potential was held at –65 and 0 mV, respectively. The amplitude of excitatory current is larger when the stimulus is moving in the preferred direction and the amplitude of inhibitory current is larger when the stimulus is moving in the null direction. D, dendritic morphology of the recorded on–off DSGC. Dendrites are distributed in two layers, and exhibit recursive branches forming loop-like structures. Arrowheads indicate tracer-coupled cells. E and F, higher magnification pictures showing on- and off-stratification of the area indicated by the box in D.
Physiological properties of P11 and adult DSGCs
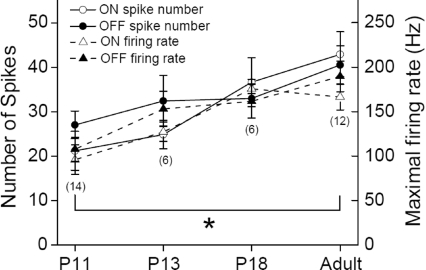
The light responses of P11 RGCs showed fewer spikes and lower firing rate, and were easily fatigued to repetitive stimuli, taking an interval of 1 min to regain the initial level of response to a subsequent stimulus. Figure 2 shows that the number of spikes in light responses increased from 21.2 ± 4.4 at P11 to 42.9 ± 5.1 in adults for the on-response, and from 27.0 ± 3.1 to 40.5 ± 4.3 for the off-response, and the maximal firing rate also increased from 95.9 ± 16.5 (on-response) and 107.5 ± 13.5 Hz (off-response) at P11 to 166.4 ± 14.5 and 189.7 ± 17.1 Hz, respectively, in adults.
Figure 2. Early postnatal DSGCs exhibited weaker light responses, as measured by the number of spikes (○ and▵) and maximal firing rate (• and ▴) to each presentation of visual stimulus (stationary spot flashing for 1 s).
Numbers in parentheses indicate number of cells. Significant differences can be observed between P11 and adult DSGCs (*P < 0.05; error bars indicate s.e.m.).
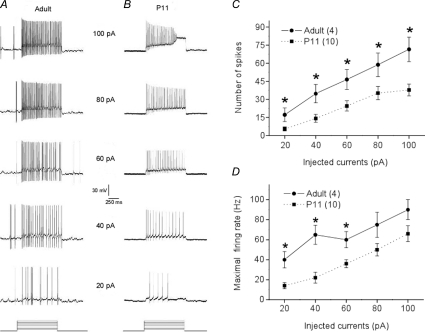
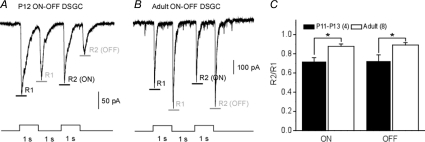
Fewer spikes and lower firing rate could result from lower membrane excitability, weaker synaptic connections and different receptor composition on the postsynaptic membrane. We tested all these possibilities. First, we performed current clamp experiments and injected different amounts of current into DSGCs. We found that P11 cells always generated fewer spikes at lower frequencies than the adult DSGCs (Fig. 3A), and spiking usually stopped owing to excitatory block when the current exceeded 100 pA (Fig. 3B). A significant difference was observed at every current intensity between P11 and adult cells (Fig. 3C and D). These results showed that the membrane excitability is lower in P11 than in adult cells. Second, we tested reproducibility of synaptic connections by examining responses to sequentially flashed spots (Fig. 4A and B). Compared with adult cells, both on- and off-responses of the early postnatal cells (P11–P13) to the second flash were much smaller in amplitude (Fig. 4C), indicating weaker and less reliable synaptic connections in early postnatal DSGCs. Furthermore, when the response kinetics was compared between age groups, the EPSCs of early postnatal DSGCs exhibited slower rising and falling phases than those of the adult DSGCs (Fig. 5A and B), indicating different receptor compositions between early postnatal cells and adult cells. However, when the kinetics of first and second responses was compared within the same age group, there was no difference in rising or falling phases (Fig. 5C). These results indicate that the mechanism for the much smaller second responses in the early postnatal cells is mainly located on the presynaptic side and is likely to result from depletion of synaptic vesicles.
Figure 3. Lower membrane excitability of P11 DSGCs.
A and B, spike traces comparing responses to the same strength of current injected into identified P11 and adult DSGCs. It is clear that the P11 DSGCs fire fewer spikes at a lower frequency. C and D, population results for number of spikes and maximal firing rate, respectively. Numbers in parentheses are number of cells. *P < 0.05 (error bars indicate s.e.m.).
Figure 4. Weaker synaptic connections.
Reproducibility of synaptic transmission was assessed by a sequential flashing light protocol. A, EPSCs of a P12 DSGC recorded at –65 mV. B, EPSCs of an adult DSGC recorded at –65 mV. Abbreviations: R1, response to first flash; and R2, response to second flash. The black and grey horizontal bars indicate the peak of on- and off-currents, respectively. C, R2/R1 ratio of on- and off-responses, comparing early postnatal and adult DSGCs. The R2/R1 ratio is much lower for early postnatal DSGCs, indicating a lower reproducibility of synaptic transmission at this early developmental stage. Numbers in parentheses indicate number of cells. *P < 0.05 (error bars indicate s.e.m.).
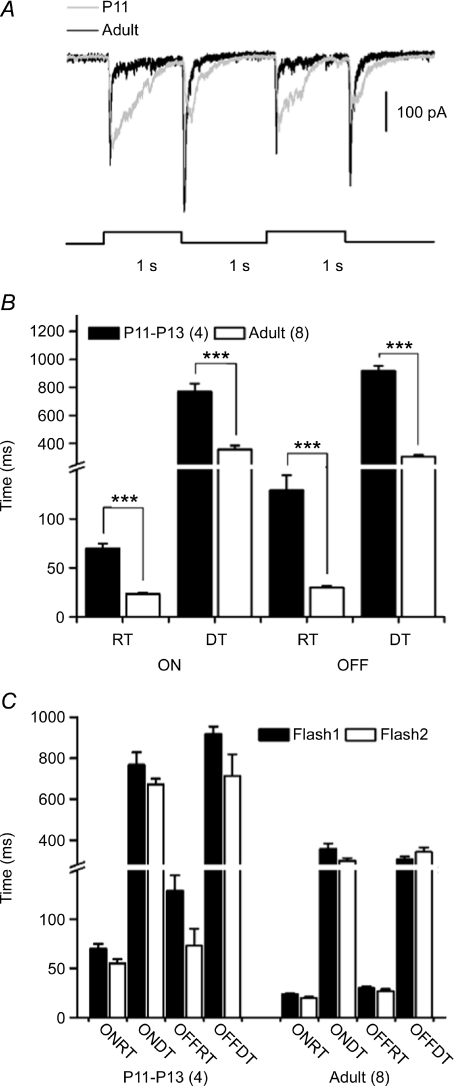
Figure 5. Slower kinetics of synaptic responses.
A, voltage clamp recordings of responses to a sequentially flashed spot from a P11 (grey trace) and an adult DSGC (black trace). B, statistical results showing comparisons of rising and falling phases of on- and off-responses between P11–P13 and adult DSGCs (***P < 0.0001). Numbers in parentheses indicate number of cells. C, comparisons of the rising and falling phases of the responses to the first and second flash within the same age group. No statistically significant difference can be seen (P > 0.05). Error bars indicate s.e.m. Abbreviations: RT, rise time; and DT, decay time.
Robust directionality at onset of light sensitivity
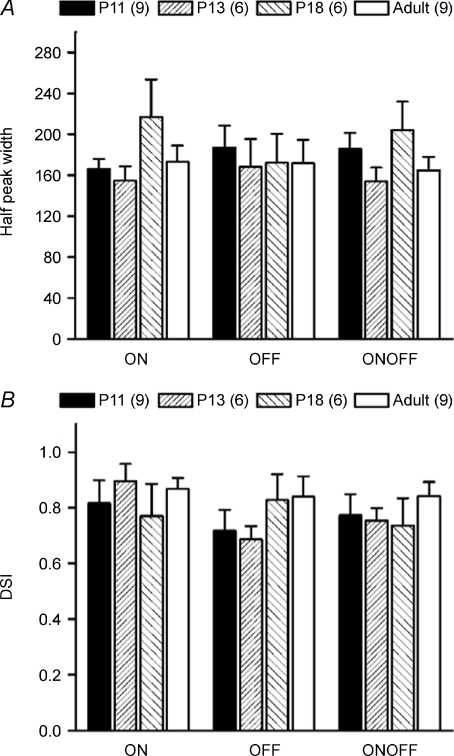
Despite the differences in membrane excitability, strength of synaptic connections and synaptic kinetics, P11 DSGCs exhibited remarkably good direction selectivity. We plotted direction tuning curves of DSGCs recorded in P11, P13, P18 and adult cells and measured the width at the half-peak rate (Fig. 6A). We also calculated the DSI on the same set of cells (Fig. 6B). There were no statistically significant differences between P11, P13, P18 and adult DGSCs (> P30) for both parameters. These findings indicate that the mechanisms for computing motion direction are present and robust as soon as the RGCs respond to light.
Figure 6. Constant direction selectivity throughout development.
The level of direction selectivity assessed by the width of the direction tuning curve at the half-peak amplitude (A) and the DSI (B) at postnatal day 11, 13 and 18 was almost identical to those measured in adult cells. Numbers in parentheses are number of cells. (P > 0.05, mean ±s.e.m.).
Light-independent development of DS circuitry
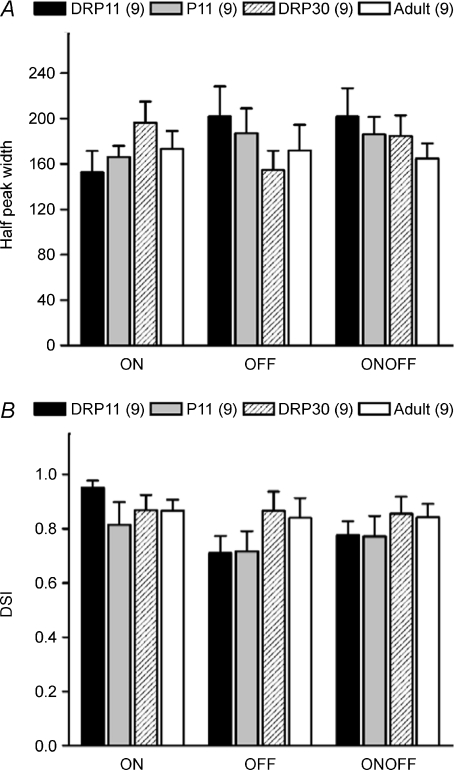
We investigated whether the formation of retinal DS circuitry is affected by light input. We reared mice in complete darkness from P0 to P11 or to P30, and systematically surveyed the RGCs in the animals reared in the dark to P30. We found that the percentage of the on–off cells was significantly increased (Supplementary Fig. 2), consistent with the previous findings in dark-reared mice (Tian & Copenhagen, 2003). This result confirmed that our dark rearing is effective. Although the percentage of on–off cells was quite different from that of Tian & Copenhagen (2003), this may have resulted from different recording techniques, since we used loose patch and they used multi-electrode array. Nevertheless, DSGCs could still be recorded from dark-reared retinae; their morphological features were very similar to those of DSGCs recorded in control retinae (Supplementary Fig. 3), and both the width of the direction tuning curve at the half-peak rate and the DSI of the dark-reared DSGCs were almost identical to those of the control DSGCs at matching ages (Fig. 7A and B). This result demonstrated that the formation of the directional circuitry is completely independent of light input, consistent with very recent findings (Chan & Chiao, 2008; Elstrott et al. 2008).
Figure 7. Dark rearing did not change direction selectivity.
The width of the direction tuning curve measured at half-peak amplitude (A) and the DSI (B) were very similar when comparing DSGCs from the retinae of mice reared in darkess to P11 or P30 with those from age-matched control retinae. Numbers in parentheses are number of cells (P > 0.05, mean ±s.e.m.).
Discussion
Two recent studies found that retinal direction selectivity is present at postnatal eye opening in the mouse using multi-electrode array (Elstrott et al. 2008) and in the rabbit using extracellular recording (Chan & Chiao, 2008). Both studies found that dark rearing did not affect the direction selectivity, consistent with earlier findings that raising rabbits in a conditioned environment did not affect retinal direction selectivity (Daw & Wyatt, 1974). Our study not only confirmed these findings using whole-cell patch clamp recording, but also advanced the emergence of retinal direction selectivity by a few days to the onset of light sensitivity. Furthermore, with patch clamp recording, we were able to show that the dendritic morphology and synaptic input patterns are not affected by light deprivation. We also showed that extensive dark rearing does not change the motion computation, indicating that the maintainance of directional circuitry is also light independent.
During the early postnatal stage, synchronous activities propagate in the retina in a wave-like fashion (Wong et al. 1995). By using a transgenic animal deficient in the nicotinic ACh receptor β2 subunit (β2−/− animals), Elstrott et al. (2008) ruled out the involvement of propagating activities mediated by ACh from P0 to P10 in producing retinal direction selectivity (Torborg & Feller, 2005). From P11, synchronous activities were mediated by glutamate (Bansal et al. 2000). Our data showed that direction-selective responses were already present at the onset of glutamate-mediated waves, and therefore ruled out the involvement of propagating activity mediated by glutamate in generating direction selectivity. Together with the results of Elstrott et al. (2008), we can conclude that neither ACh- nor glutamate-mediated waves are involved in formation of the neural circuitry underlying retinal direction selectivity. However, these experiments have not ruled out involvement of propagating activity, since there are curare-insensitive propagating activities in AChR β2−/– animals as shown by Bansal et al. (2000).
A difference between our study and that of Elstrott et al. (2008) is that we found the width of the direction tuning curve from P11 DSGCs to be the same as that of adult cells. The reason may lie in the difference in visual stimulus; we used a drifting bar with an interstimulus interval of 1 min, whereas Elstrott and colleagues used drifting gratings with interstimulus interval of 10 s. Therefore, we tested peak responses and they tested adapted responses. Since we found that the early postnatal cells are adapting more dramatically to repeated stimuli, one would expect the continuous drifting gratings will lead to a lower amplitude of preferred response and a smaller directional index in early postnatal cells.
In the rabbit and mouse retina, DSGCs have been identified at postnatal eye opening (Masland, 1977; Chan & Chiao, 2008; Elstrott et al. 2008). However, RGCs acquire light sensitivity several days before eye opening, and light through the eyelid has been shown to drive the lateral geniculate and cortical neurons (Krug et al. 2001; Akerman et al. 2002). We show here that at the onset of light sensitivity, early postnatal DSGCs exhibited the same selectivity for a moving stimulus as the adult DSGCs, despite possessing many different physiological properties, such as lower membrane excitability, slower light response kinetics and lower reproducibility to repeated stimuli. In the mouse retina, the electron microscopic detection of the earliest ribbon synapses in the inner plexiform layer is at P10 (Olney, 1968; Fisher, 1979) and the onset of expression of the vesicular glutamate transporter VGluT1 is slightly earlier (Sherry et al. 2003; Wassle et al. 2006), so the DSGCs exhibited adult-like responses almost as soon as they formed synapses with bipolar cells. The presence of robust direction selectivity at the onset of light sensitivity and the light-independent formation of the DS circuitry strongly suggest that the DS circuitry, in other words, the connection between DSGCs and SACs, forms before the RGCs receive bipolar cell inputs.
The RGC membrane excitability has been examined in the cat (Skaliora et al. 1993), rat (Wang et al. 1997) and mouse (Rothe et al. 1999) during development. Our findings are very consistent with the previous reports from all these species. The only difference in our case is that the comparison was made in a morphologically and physiologically characterized subtype of RGCs.
The identical kinetics of the first and second response indicates that the reduced second response is due to the reduced input from the presynaptic terminals rather than a change in postsynaptic composition as described in many paired pulse experiments (Manabe et al. 1993). In principle, synaptic connections, both between photoreceptors and bipolar cells and between bipolar cells and RGCs, could account for this reduction. The synapses between photoreceptors and bipolar cells form before those from bipolar cells to RGCs (Olney, 1968; Sherry et al. 2003; Weng et al. 2005; Wassle et al. 2006) and are the less likely possibility, although our evidence does not allow a definitive conclusion.
The response kinetics is much slower in early postnatal DSGCs. In tectal neurons, the AMPA/NMDA ratio increases as the animals develop, leading to faster response kinetics (Wu et al. 1996). However, there is hardly any AMPA receptor expression in adult on–off DSGCs (Kittila & Massey, 1997), so the underlying mechanism for this decrease in rising and falling phases as the DSGCs mature cannot be due to a similar change in the AMPA/NMDA ratio. A recent study showed that deletion of synaptotagmin 2 slows down the EPSC kinetics in the calyx-of-Held synapse (Sun et al. 2007), providing a possibility that the presynaptic mechanisms responsible for synchronized release develop as the synapses mature.
Zhang and co-workers showed that more SACs undergo apoptosis under dark-rearing condition (Zhang et al. 2005). However, three studies examining the effect of dark rearing on direction selectivity, including ours, did not find any change caused by light deprivation. The reason might be that there is quite a large redundancy in SACs coverage, as calculated by Dong et al. (2004) in the rabbit retina. Given a 30% loss of SACs, there are still four to seven SACs to cover each DSGC. Therefore, the loss of SACs may not be severe enough to affect direction selectivity.
The mechanism of connecting direction-selective circuitry is still not clear. The simplest hypothesis for formation of direction-selective circuitry is for each subtype of DSGC to express a particular adhesion molecule that interacts with the same adhesion molecule expressed on the appropriate sector of SACs. However, there is no such evidence that the SACs express different adhesion molecules on different sectors or that different subtypes of DSGCs express different adhesion molecules. Further experiments are required to test this hypothesis.
Acknowledgments
This project was supported by an National Natural Science Foundation of China key project grant (30530280) and two grants from the National Basic Research Program of China (2007CB512208 and 2006CB911003) to S.H. We thank Professor W. R. Levick for helpful comments on scientific contents and on style and grammar of English, Yingye Zhang, Zhiping Liu and Hai-tian Liang for technical support.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.161240/DC1
References
- Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/s0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe-Anders C, Miller RF, Dacheux R. Developmental characteristics of receptive organization in the isolated retina-eyecup of the rabbit. Brain Res. 1975;87:61–65. doi: 10.1016/0006-8993(75)90779-9. [DOI] [PubMed] [Google Scholar]
- Brecha N, Johnson D, Peichl L, Wassle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and γ-aminobutyrate immunoreactivity. Proc Natl Acad Sci USA. 1988;85:6187–6191. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Chiao CC. Effect of visual experience on the maturation of ON-OFF direction selective ganglion cells in the rabbit retina. Vision Res. 2008;48:2466–2475. doi: 10.1016/j.visres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Coombs JL, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. J Comp Neurol. 2007;503:803–814. doi: 10.1002/cne.21429. [DOI] [PubMed] [Google Scholar]
- Coombs J, Van Der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Daw NW, Wyatt HJ. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J Physiol. 1974;240:309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer DJ, Vaney DI. Gap-junction communication between subtypes of direction-selective ganglion cells in the developing retina. J Comp Neurol. 2005;482:85–93. doi: 10.1002/cne.20351. [DOI] [PubMed] [Google Scholar]
- Diao L, Sun W, Deng Q, He S. Development of the mouse retina: emerging morphological diversity of the ganglion cells. J Neurobiol. 2004;61:236–249. doi: 10.1002/neu.20041. [DOI] [PubMed] [Google Scholar]
- Dong W, Sun W, Zhang Y, Chen X, He S. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J Physiol. 2004;556:11–17. doi: 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Fisher LJ. Development of retinal synaptic arrays in the inner plexiform layer of dark-reared mice. J Embryol Exp Morphol. 1979;54:219–227. [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 2007;5:e185. doi: 10.1371/journal.pbio.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Dong W, Deng Q, Weng S, Sun W. Seeing more clearly: recent advances in understanding retinal circuitry. Science. 2003;302:408–411. doi: 10.1126/science.1085457. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997;77:675–689. doi: 10.1152/jn.1997.77.2.675. [DOI] [PubMed] [Google Scholar]
- Kong JH, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed lids. J Neurophysiol. 2001;85:1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Gibo TL, Grzywacz NM. Dark-rearing-induced reduction of GABA and GAD and prevention of the effect by BDNF in the mouse retina. Eur J Neurosci. 2006;24:2118–2134. doi: 10.1111/j.1460-9568.2006.05078.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron. 2006;51:787–799. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Masland RH. Maturation of function in the developing rabbit retina. J Comp Neurol. 1977;175:275–286. doi: 10.1002/cne.901750303. [DOI] [PubMed] [Google Scholar]
- Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968;7:250–268. [PubMed] [Google Scholar]
- Rothe T, Jüttner R, Bähring R, Grantyn R. Ion conductances related to development of repetitive firing in mouse retinal ganglion neurons in situ. J Neurobiol. 1999;38:191–206. [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Scobey RP, Chalupa LM. Prenatal development of excitability in cat retinal ganglion cells: action potentials and sodium currents. J Neurosci. 1993;13:313–323. doi: 10.1523/JNEUROSCI.13-01-00313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wang GY, Ratto G, Bisti S, Chalupa LM. Functional development of intrinsic properties in ganglion cells of the mammalian retina. J Neurophysiol. 1997;78:2895–2903. doi: 10.1152/jn.1997.78.6.2895. [DOI] [PubMed] [Google Scholar]
- Wassle H, Regus-Leidig H, Haverkamp S. Expression of the vesicular glutamate transporter vGluT2 in a subset of cones of the mouse retina. J Comp Neurol. 2006;496:544–555. doi: 10.1002/cne.20942. [DOI] [PubMed] [Google Scholar]
- Weng S, Sun W, He S. Identification of ON–OFF direction-selective ganglion cells in the mouse retina. J Physiol. 2005;562:915–923. doi: 10.1113/jphysiol.2004.076695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Wu ML, Chiao CC. Light deprivation delays morphological differentiation of bipolar cells in the rabbit retina. Brain Res. 2007;1170:13–19. doi: 10.1016/j.brainres.2007.06.091. [DOI] [PubMed] [Google Scholar]
- Xu HP, Tian N. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening. J Comp Neurol. 2007;503:244–259. doi: 10.1002/cne.21379. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Wu SM. Development of cholinergic amacrine cells is visual activity-dependent in the postnatal mouse retina. J Comp Neurol. 2005;484:331–343. doi: 10.1002/cne.20470. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Lee S. Direction-selective light responses of DS ganglion cells at the onset of vision. Invest Ophthalmol Vis Sci. 2005;46:2242–. [Google Scholar]
- Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.