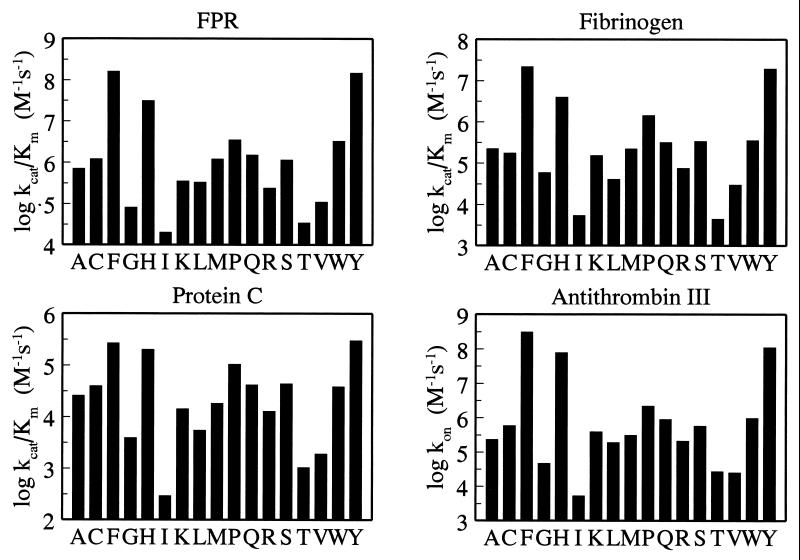
Figure 2.
Effect of residue 225 on thrombin interactions. Plotted are the values (in log units) of kcat/Km for the hydrolysis of H-D-Phe-Pro-Arg-p-nitroanilide (FPR), fibrinogen, and protein C (in the presence of 5 mM CaCl2 and 100 nM rabbit thrombomodulin), and the kon values for inhibition of thrombin by antithrombin III (in the presence of 0.5 United States Pharmacopeia units/ml of heparin). Experimental conditions are: 5 mM Tris, 145 mM NaCl, 0.1% polyethylene glycol, pH 7.4 at 37°C (see legend to Fig. 1 for FPR). Measurements of Km and kcat for FPR hydrolysis by several mutants show that the reduced specificity is the result almost entirely of increased Km and compromised substrate binding. The four profiles are quite similar and suggest that perturbation of residue 225 affects the environment of the primary specificity site to which FPR, fibrinogen, protein C, and antithrombin III make contact.