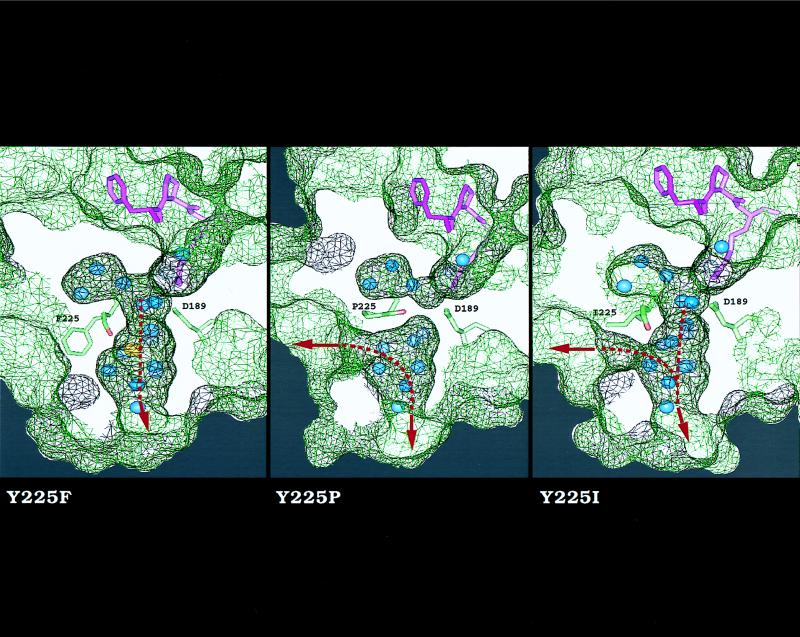
Figure 3.
Effect of residue 225 on the architecture of the water channel around the primary specificity site of thrombin. Shown is a cross section of the enzyme along the water channel that reveals the active site inhibitor PPACK (purple), D189 in the primary specificity site, residue 225 with the carbonyl O atom of residue 224 (red), buried water molecules (blue), and Na+ (yellow). The surface of the enzyme is rendered as a net (black, above the plan of section; green, below it). The side chain of residue 225 points away from D189 and makes no contact with PPACK. The Y225F mutant is practically identical to wild type (11) and shows a bound Na+ coordinated octahedrally by the carbonyl O atoms of K224 and R221a (not shown), and four water molecules (23, 26). In this mutant, the water channel connects the active site to an aperture at the bottom of the molecule (arrow). In the Y225P mutant, there is no evidence of bound Na+; the carbonyl O atom of K224 is shifted 70° toward the interior of the channel and occludes it in the middle. In addition, the channel is shunted laterally (arrow) around residue 225 because of the Y225P replacement. In the Y225I mutant, the channel has three apertures.