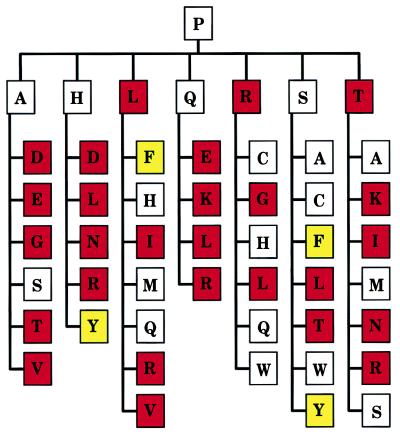
Figure 4.
Evolutionary transitions from P225 in the ancestral serine protease. Shown are the seven residues (in the single-letter code) one mutational step away from Pro in the genetic code (Ala, His, Leu, Gln, Arg, Ser, and Thr), and those one mutational step away from these residues, altogether defining 42 distinct pathways two mutational steps away from Pro. Residues are color-coded based on the associated catalytic activity toward FPR (see Fig. 2). White, residues causing less than 10-fold change in activity compared with Pro. Red, residues causing more than 10-fold loss of activity compared with Pro. Yellow, residues causing more than 10-fold gain in activity compared with Pro. Asn, Asp, and Glu were assumed to significantly compromise catalytic activity and function because the respective mutants of Y225 failed to express in quantities sufficient for characterization. Analysis of trace amounts of Y225D mutant reveals >100-fold reduction in catalytic activity relative to Pro. Furthermore, Asn, Asp, and Glu are not documented at position 225 in serine proteases. If conservation or enhancement of catalytic activity is assumed as an evolutionary criterion for transition, then Ser (with a TCN codon) would have provided the safest and most flexible intermediate on the pathway from Pro to residues with enhanced activity like Tyr and Phe.