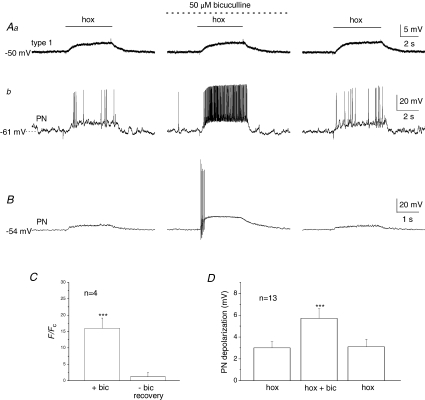
Figure 4. Bicuculline selectively potentiates the postsynaptic hypoxia-evoked chemosensory response in carotid body co-cultures.
Simultaneous gramicidin perforated-patch recordings were obtained from a presynaptic type I cell (that was part of a cluster) (Aa), and an adjacent petrosal neurone (PN) (Ab). Note depolarizing responses to hypoxia (hox; PO2∼ 5 mmHg) were recorded in both the type I cell (Aa, left trace) and the functional PN (Ab, left trace), where suprathreshold responses were evoked. Interestingly, in the presence of the GABAA receptor blocker, bicuculline (50 μm), there was a marked potentiation of the PN response resulting in a robust increase in spike discharge (Ab, middle trace), whereas the type I cell response was unaltered (Aa, middle trace). Reversibility of these responses following wash-out of the drug is shown in Aa and b (right traces). Summary data from 4 similar PNs are shown in C, where hypoxia-induced PN spike frequency (F) during bicuculline (bic) and after wash-out (recovery) is plotted as a ratio relative to initial control frequency (Fc); note the ratio F/Fc was significantly augmented (∼15×) during bicuculline, compared to after bicuculline (∼1), i.e. recovery (***P < 0.001; Mann–Whitney U test). B, another representative example from a different co-cultured PN, where only the PN postsynaptic response was recorded and was initially subthreshold; note the marked enhancement of the response in the presence of bicuculline (middle trace). D, summary data of the mean (±s.e.m.) postsynaptic depolarization before, during and after bicuculline from 13 similar PNs; hypoxia-evoked (hox) responses in the presence of bicuculline were significantly different from control hypoxic response (***P < 0.01; Student's t test).