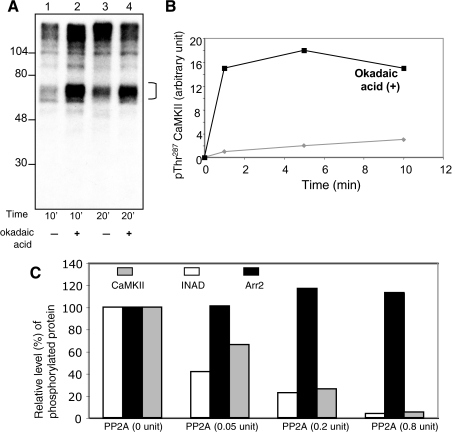
FIGURE 3.
Role of PP2A to catalyze the dephosphorylation of CaMKII and Arr2. A, potentiation of CaMKII autophosphorylation by okadaic acid. Shown is the autoradiography of an in vitro phosphorylation of fly head homogenates in the presence of Ca2+ for either 10 (lanes 1 and 2) or 20 min (lanes 3 and 4), as indicated. The effect of okadaic acid (10 nm) to potentiate autophosphorylation of CaMKII (bracket) was investigated (lanes 2 and 4). B, time-dependent potentiation of autophosphorylation of CaMKII by okadaic acid. The levels of phosphorylated CaMKII with (filled square) or without okadaic acid were determined by Western blotting using Thr(P)287-specific antibodies. Okadaic increased autophosphorylation of CaMKII at Thr287 by 15-fold following 1 min of incubation. C, PP2A dephosphorylates CaMKII, but not Arr2. Purified PP2A was incubated with phosphorylated Arr2305–400 or CaMKII for 20 min, and the remaining phosphorylated proteins were quantitated following SDS/PAGE and shown in a histogram. Phosphorylated INAD containing the first and second PDZ domains was used as a positive control for PP2A.