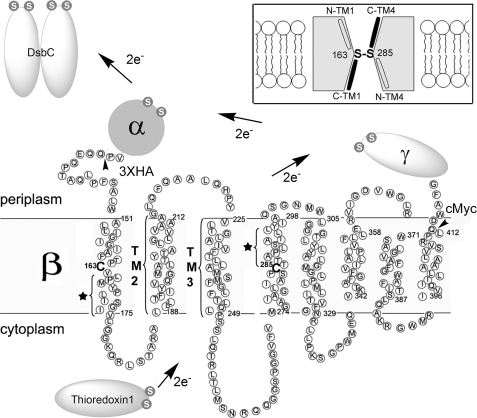
FIGURE 1.
Electron transfer pathway through transmembrane domain (β) of DsbD and its membrane topology predicted from the primary sequence. The topology of DsbDβ was predicted using HMMTOP. The essential two cysteines are shown in bold without a circle and numbered. The residues indicated with a star in TM1 and TM4 are water-exposed when the Cys-163 and Cys-285 are disulfide-bonded (19). Studies on the residues in TM2 and TM3 are shown in Fig. 4. The essential cysteines in the other domains (α and γ) and interacting proteins (Trx1 and DsbC) are shown in a white S (the sulfur of thiol) in gray circles. The tailless arrows indicate where a signal sequence of DsbD and three hemagglutinin (HA) epitopes are fused at the N terminus of DsbDβ, and a c-Myc epitope is fused at the C terminus of DsbDβ. The figure in the inset describes the model of DsbDβ, in which Cys-163 and Cys-285 form a disulfide bond in the middle of the protein and halves of C-terminal TM1 and TM4 are water-exposed (black; C-TM1 and C-TM4), whereas those of N-terminal ones are not (N-TM1 and N-TM4).