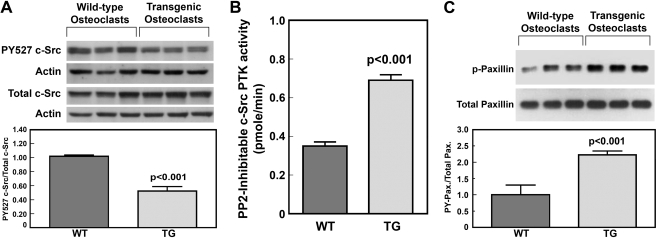
FIGURE 9.
Comparison of the PY-527 levels of c-Src (A), the cellular PP2-inhibitable c-Src PTK activity (B), and protein-tyrosine-phosphorylated (PY) levels of paxillin (C) in marrow-derived osteoclasts of young adult male transgenic mice with those in osteoclasts of age- and sex-matched WT littermates. A, cell extracts of osteoclasts derived from three male transgenic mice and three WT littermates were separated on SDS-PAGE and blotted with anti-Tyr(P)527 c-Src and anti-actin antibodies. A replicate gel was blotted against total c-Src and actin. Top, Western blots. Bottom, ratio of PY-527 c-Src/actin to total c-Src/actin (mean ± S.E., n = 3). B, the total and PP2-inhibitable PTK activity of immunoprecipitated c-Src protein of transgenic and WT osteoclasts were assayed as described under “Experimental Procedures.” Greater than 95% of the PTK activity in the immunoprecipitate was inhibited by PP2. Results are shown as PP2-inhibitable c-Src PTK activity (mean ± S.E., n = 4). C, the total and Tyr(P) levels of paxillin in osteoclast extracts of three pairs of PTP-oc transgenic mice and wild-type littermates were analyzed by the Western blot using the respective specific antibodies. Top, Western blots; bottom, ratio of protein-tyrosine phosphorylated paxillin to total paxillin (mean ± S.E., n = 3).