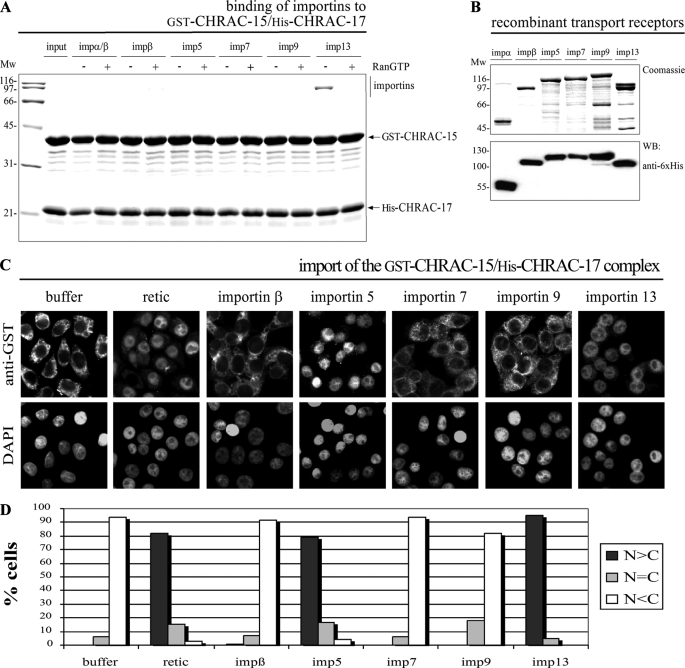
FIGURE 1.
Accumulation of the CHRAC-15/17 heterodimer in the nucleus of permeabilized cells is mediated by importin 5 and importin 13. A, GST-CHRAC-15 and His-CHRAC-17 were coexpressed in E. coli and immobilized on glutathione-Sepharose. The CHRAC-15/17 complex was incubated with equal concentrations (∼0.2 μm) of recombinant purified importin α/β dimer, importin β, importin 5, importin 7, importin 9, or importin 13. Binding was performed in the absence (-) or presence (+) of RanGTP (Q69L mutant) to simulate cytoplasmic and nuclear conditions, respectively. Bound fractions were analyzed by SDS-PAGE followed by Coomassie staining. The CHRAC-15/17 heterodimer binds predominantly to importin 13 in a RanGTP-sensitive manner. Binding of other nuclear transport receptors was not detected. B, the transport receptors importin α, importin β, importin 5, importin 7, importin 9, and importin 13 used for in vitro studies were expressed in E. coli, analyzed by SDS-PAGE, and either stained with Coomassie or analyzed by Western blot using anti-His6 antibody. C, digitonin-permeabilized HeLa P4 cells were incubated with 0.4 μm purified GST-CHRAC-15/His-CHRAC-17 heterodimer, reticulocyte lysate (retic), or the indicated nuclear transport receptors (∼0.4 μm each), a RanGDP/NTF2 mix, and an energy regenerating system for 30 min at 37 °C. For a negative control, reticulocyte lysate was replaced by transport buffer (buffer). The cells were fixed, and the subcellular localization of the CHRAC-15/17 complex was visualized by indirect immunofluorescence (anti-GST). The DNA was counterstained using 4′,6-diamidino-2′-phenylindole. D, the mean distribution of the CHRAC-15/CHRAC-17 heterodimer was quantified for 50 cells/condition in the three categories: N>C, N=C, and N<C. Mw, molecular mass; imp, importin; WB, Western blot; DAPI, 4′,6′-diamino-2-phenylindole.