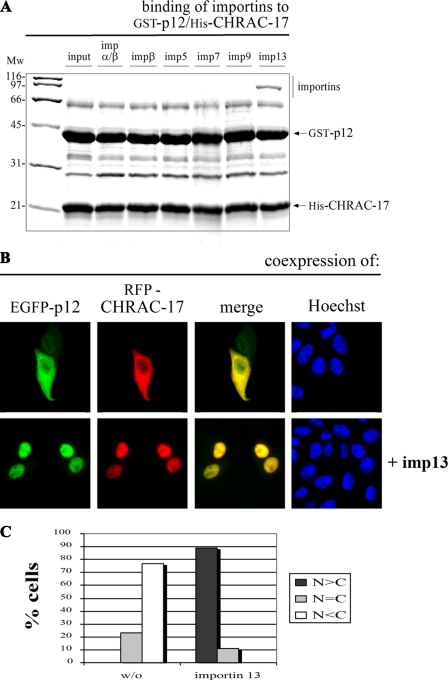
FIGURE 4.
Importin 13 also facilitates the nuclear accumulation of the p12/CHRAC-17 heterodimer. A, GST-p12 and His-CHRAC-17 were coexpressed in E. coli, immobilized on glutathione-Sepharose, and incubated with equal concentrations (0.2 μm) of recombinant purified importin α/β heterodimer, importin β, importin 5, importin 7, importin 9, or importin 13. Bound fractions were analyzed by SDS-PAGE followed by Coomassie staining. The histone fold heterodimer p12/CHRAC-17 binds exclusively to importin 13. B, HeLa P4 cells were transiently cotransfected with plasmid DNA coding for EGFP-p12 and RFP-CHRAC-17 (top row) and additional expression of FLAG-tagged importin 13 (bottom row). The localization of the EGFP-p12 and RFP-CHRAC-17 fusion proteins was determined 24 h post-transfection by direct fluorescence. Colocalization of p12 and CHRAC-17 is shown in yellow (merge). The DNA was counterstained with Hoechst. The largely cytoplasmic distribution of the p12/CHRAC-17 heterodimer was abolished in the presence of FLAG-importin 13, leading to a strong accumulation in the cell nucleus. C, for a semi-quantitative analysis, the mean distribution of the EGFP-p12/RFP-CHRAC-17 heterodimer with or without the additional coexpression of importin 13 was classified in the categories N>C, N=C, and N<C. Mw, molecular mass; imp, importin.