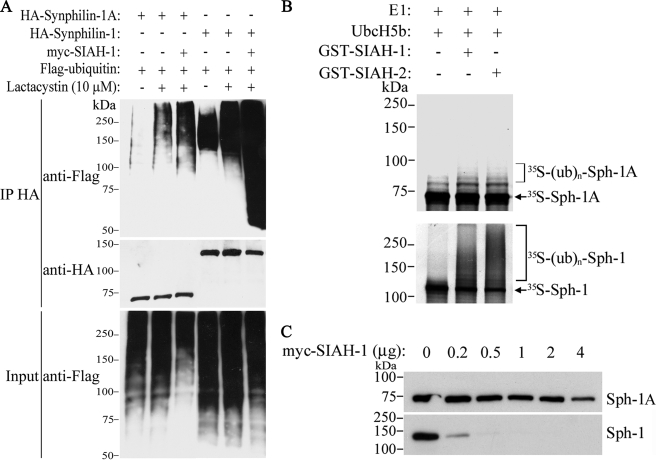
FIGURE 2.
SIAH promotes slight ubiquitylation and degradation of synphilin-1A. A, HEK293 cells were co-transfected with HA-synphilin-1A, FLAG-ubiquitin, in the absence or in the presence of myc-SIAH-1. As control, co-transfections were carried out with synphilin-1 instead of synphilin-1A. Cells were incubated 12 h with 10 μm lactacystin, and HA-synphilin-1A and HA-synphilin-1 were immunoprecipitated (IP) with anti-HA antibody. Ubiquitylation of synphilin-1A and synphilin-1 was detected by Western blot using anti-FLAG antibody. The middle panel shows the levels of immunoprecipitated synphilin-1A and synphilin-1 using anti-HA antibody. The lower panel shows the total ubiquitylation levels of co-transfected cells with anti-FLAG antibody. B, in vitro translated 35S-synphilin-1A was incubated with recombinant SIAH-1 or SIAH-2, UbcH5b, ubiquitin, and the other purified components of the ubiquitin system. The levels of 35S-synphilin-1A in vitro ubiquitylation were determined by PhosphorImager analysis (upper panel). As control, in vitro translated 35S-synphilin-1 was incubated with recombinant SIAH-1 or SIAH-2 under the same conditions as for 35S-synphilin-1A, and the levels of 35S-synphilin-1 ubiquitylation were determined by PhosphorImager analysis (lower panel). E1, ubiquitin-activating enzyme. C, effect of SIAH-1 on synphilin-1A (Sph-1A) steady-state levels. HEK293 cells were transfected with HA-synphilin-1A and myc-SIAH-1. HA-synphilin-1A and HA-synphilin-1 from total cell lysates were detected by Western blot using anti-HA antibody.