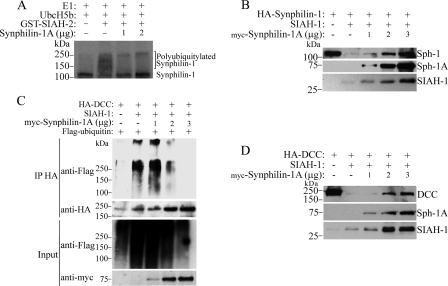
FIGURE 7.
Synphilin-1A decreases the ubiquitylation and degradation of the SIAH-1 substrates synphilin-1 and DCC. A, in vitro translated synphilin-1 was incubated with increasing amounts of synphilin-1A, UbcH5b, ubiquitin, and the other purified components of the ubiquitin system, in the absence or in the presence of SIAH-1. The levels of synphilin-1 ubiquitylation were determined by PhosphorImager analysis. E1, ubiquitin-activating enzyme. B, HEK293 cells were transfected with HA-synphilin-1, untagged SIAH-1, and increasing amounts of synphilin-1A. HA-synphilin-1 from total cell lysates was detected by Western blot using anti-HA antibody (upper panel). The expression levels of synphilin-1A (Sph-1A) and SIAH-1 were determined with anti-Myc and anti-SIAH-1 (N-15) antibodies, respectively. C, HEK293 cells were transfected with HA-DCC and increasing amounts of myc-synphilin-1A. Cells were incubated 12 h with 10 μm lactacystin. HA-DCC was immunoprecipitated with anti-HA antibody, and ubiquitylated DCC was detected by Western blot using anti-FLAG antibody. The amount of immunoprecipitated DCC was determined using anti-HA antibody (2nd panel). The levels of synphilin-1A were determined with anti-Myc antibody (4th panel). D, HEK293 cells were transfected with HA-DCC, untagged SIAH-1, and increasing amounts of synphilin-1A. HA-DCC from total cell lysates was detected by Western blot using anti-HA antibody (upper panel). The expression levels of synphilin-1A and SIAH-1 were determined with anti-Myc and anti-SIAH-1 (N-15) antibodies, respectively.