Figure 1.
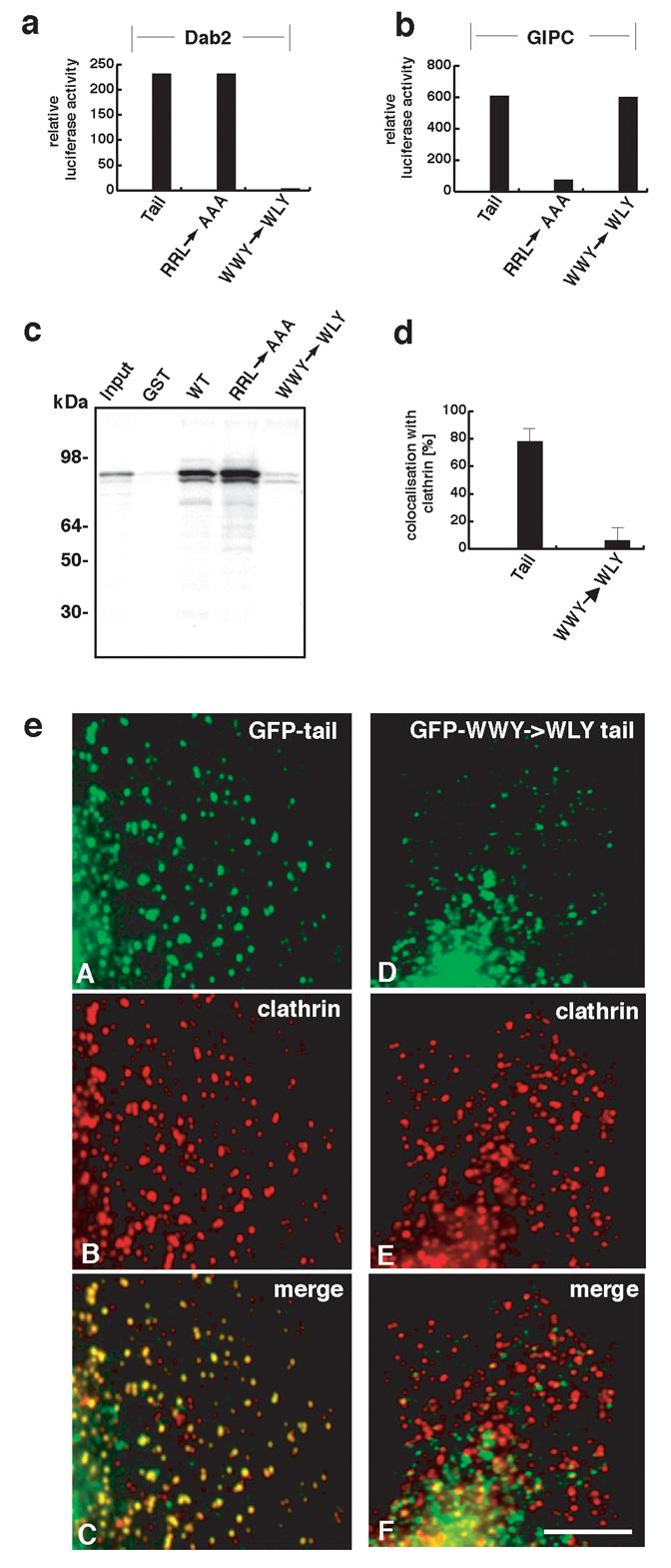
Two distinct “hot spots” for binding Dab2 and GIPC were identified on the myosin VI tail and it is shown that an intact Dab2 binding site is required for targeting myosin VI to CCSs. (a) and (b) Deletion and mutated constructs of the chicken myosin VI tail were screened for binding to Dab2 and GIPC using the mammalian 2-hybrid system. The mutation W1184L (WWY→WLY) in the tail abolishes binding to Dab2 whilst preserving binding to GIPC. Mutation of the GIPC binding site (amino acids 1107-1109, RRL→AAA) abolishes GIPC binding in addition to optineurin binding but has no effect on Dab2 binding. These results indicate that there are two distinct sites for binding partners on the myosin VI tail. (c) Autoradiograph of [35S]Dab2 “pull-down” fractions. Recombinant, purified GST-tagged myosin VI tail containing the WWY→WLY mutation does not bind [35S]Dab2 whereas GST-tagged myosin VI tail (both wild-type and containing the mutations for GIPC/optineurin binding (RRL→AAA) show binding to [35S]Dab2. GST alone was included as a control. (d) Quantitation of the colocalisation of endogenous clathrin with GFP-tagged myosin VI tail (+ LI) (both wild type and Dab2 binding mutant) by measuring the immunofluorescence staining shown in (e). Myosin VI containing the mutated Dab2 binding site (WWY→WLY) does not colocalise with clathrin. (e) Immunofluorescence staining of over-expressed GFP-labelled myosin VI whole tail (+LI) constructs and endogenous clathrin in HeLa cells. Panels A and D show the GFP-labelled wild-type tail and GFP-tail containing WWY→WLY mutation respectively. Panels B and E show the endogenous clathrin staining and C and F show the merged images. Dab2 binding is required for targeting the myosin VI isoform with the large insert (+LI) to clathrin-coated pits and vesicles. Note the tail with the WWY→WLY mutation localises to punctate structures but since they are not clathrin-coated vesicles they are probably secretory or uncoated vesicles. Scale bar = 5 μm.
