Figure 4.
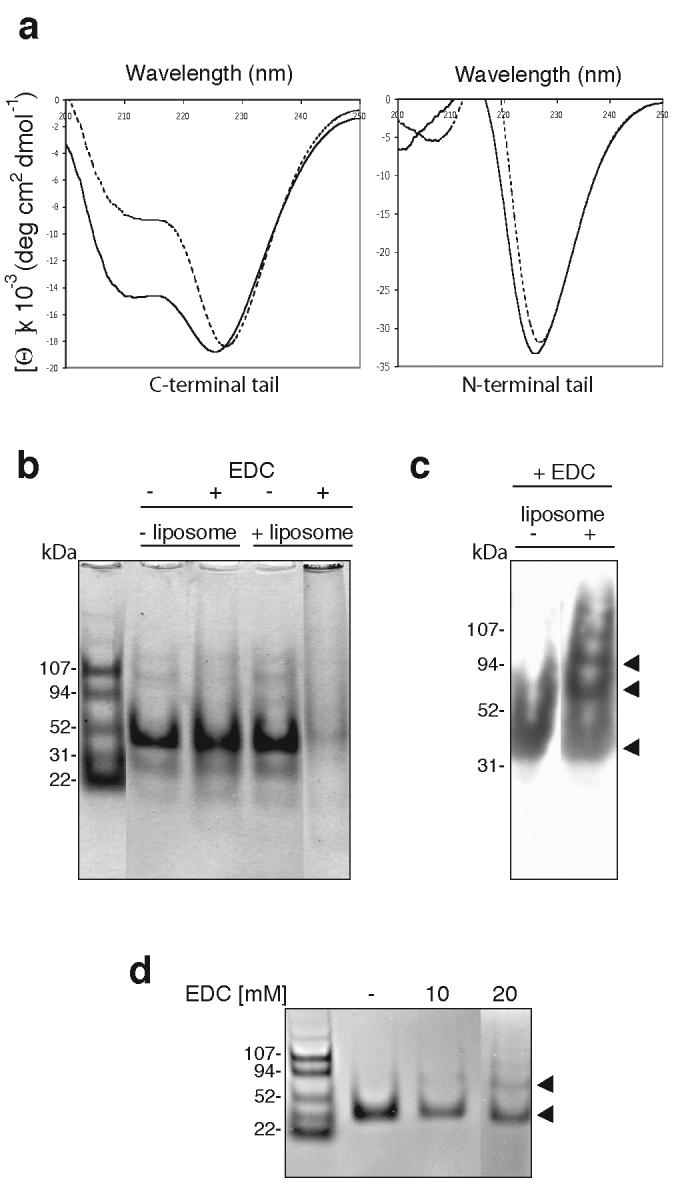
Liposome binding increases the helical structure of the C-terminal tail of myosin VI and induces dimerisation. (a) Circular dichroic spectra of the myosin VI C-terminal tail (CT) and the N-terminal tail with (solid lines: + 0.2 mg ml-1 liposomes) or without mixed brain liposomes (dashed lines). An average of five spectra are shown, normalised against spectra of buffer alone or buffer + liposomes. With liposomes CT adopts a highly helical spectrum and the (helical) signal at 222nm increases by 31% whereas the spectrum of the N-terminal tail is not altered (this construct does not bind liposomes in sedimentation assays: see figure 2c). (b) Liposome binding induces multimerisation of the myosin VI C-terminal tail. 12 μM CT was incubated with or without 0.4 mg ml-1 mixed brain liposomes and/or 90 mM EDC, a zero-length crosslinker, and analysed by SDS-PAGE. Without liposomes the monomer CT tail (30kDa) is not crosslinked by EDC but with both liposomes and EDC, the monomer band multimerises and remains at the top of the gel. BSA was used as a negative control and was not crosslinked upon addition of liposomes and EDC (data not shown). (c) Western blot of the C-terminal tail (+/− liposomes, + 90 mM EDC) using a polyclonal myosin VI C-terminal antibody. With liposomes, only a monomer band is visible whereas without liposomes, dimer, tetramer (indicated by the arrows) and trace higher order bands can be detected. (d) Liposome binding induces dimerisation of the myosin VI C-terminal tail (CT). The SDS-PAGE gel shows CT (12 μM) after a one-hour incubation with 0.4 mg ml-1 mixed brain liposomes and: lane 1, no EDC; lane 2, 10 mM EDC; lane 3, 20 mM EDC. The positions of monomer and dimer bands are indicated with black arrowheads.
