Figure 5.
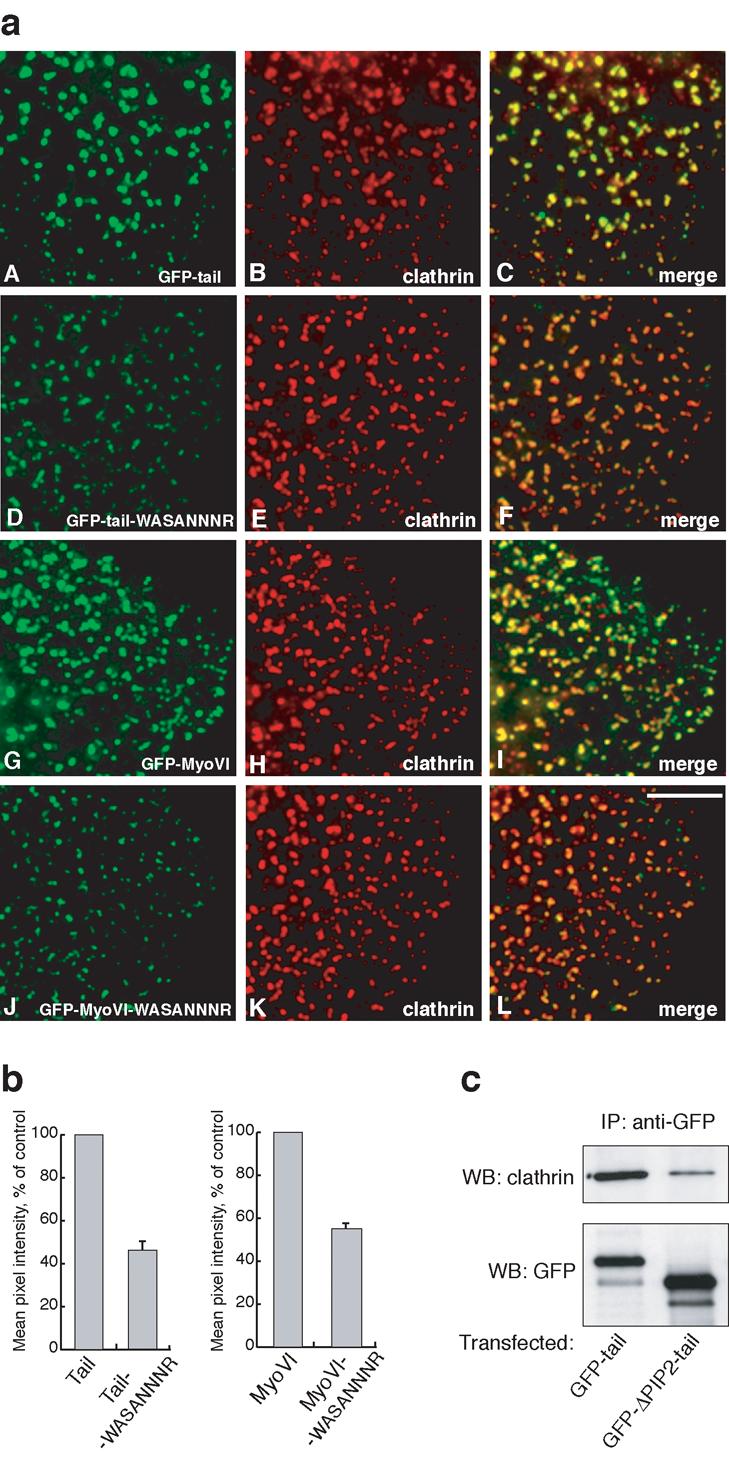
Mutations in the PIP2 binding site reduce the targeting of myosin VI to clathrin-coated structures in vivo. In a) HeLa cells were transfected with full-length wild-type GFP-myosin VI (G-I) or GFP-tail (A-C) or mutants (J-L and D-F respectively) containing the –WASANNNR- point mutation in the PIP2 binding site and processed for immunofluorescence with anti-GFP and anti-clathrin antibodies. Both tail domain and full-length myosin VI with mutations in the PIP2 binding site show significantly reduced recruitment to clathrin-coated structures at the plasma membrane (F and L). The fluorescence intensity of the different GFP-labelled constructs on at least 500 individual CCSs was measured using IP-Lab software. Scale bar = 5 μm. In b) the bar graphs show the mean pixel intensity of GFP fluorescence on CCSs of the mutant constructs relative to the wild-type controls. The results shown are the mean values of two independent experiments (± range). In a separate experiment c) HeLa cells were transfected with intact GFP-tagged myosin VI tail or GFP-tails with a 75aa deletion of the PIP2 binding region (ΔPIP2-tail) and the expressed GFP-proteins were immunoprecipitated with anti-GFP antibodies. The immunoprecipitated (IP) complexes were run on SDS-PAGE and blotted with antibody against clathrin (top panel). One fifth of each of the IP samples were run on the same gel and blotted with GFP antibody to ensure that equal over-expression and immunoprecipitation of both tail constructs had occurred (bottom panel).
