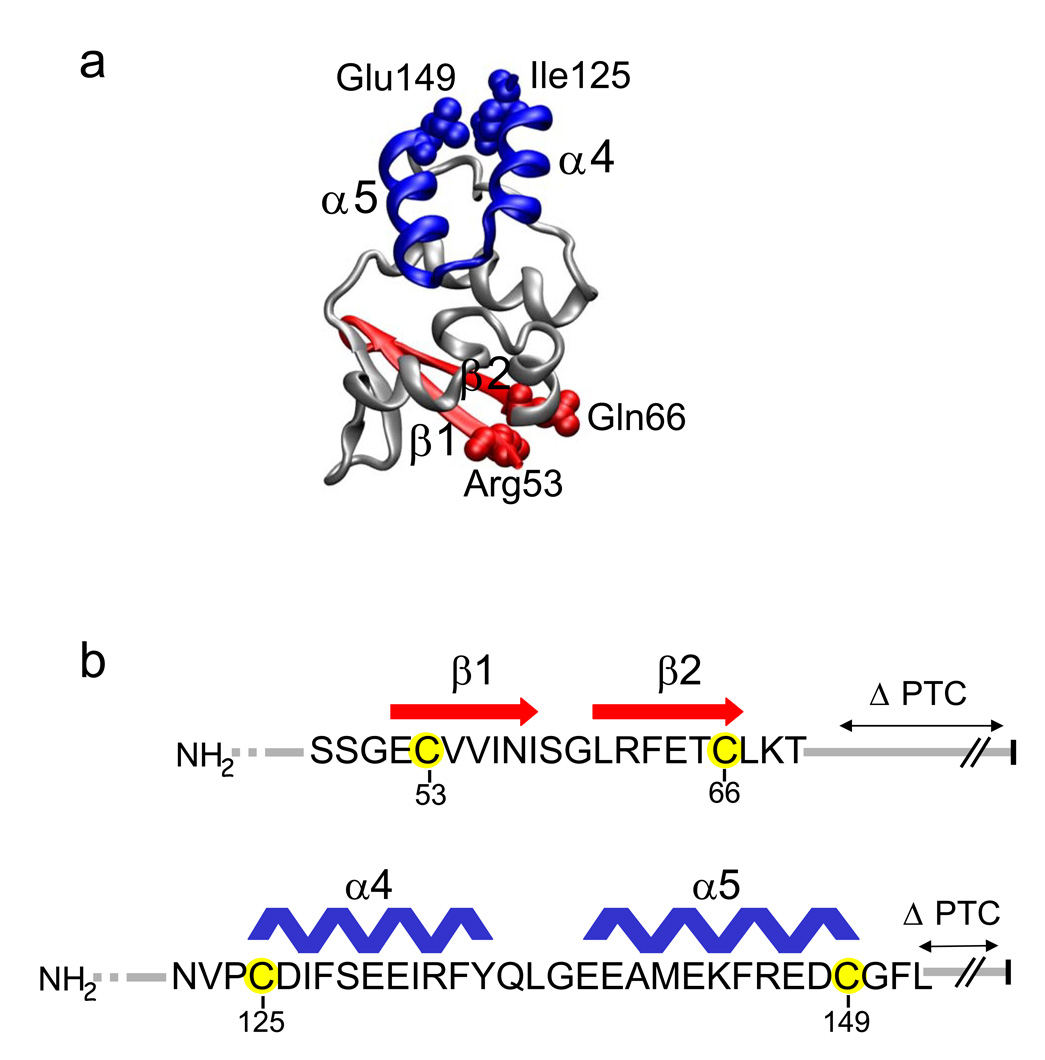
Figure 1. The T1 domain and Experimental Design.
(a) Hairpin structures. The monomeric T1 domain (taken from Minor et al., 200013 for Kv1.2) is shown with the indicated subdomains: a β–hairpin (red) comprised of β1 and β2, and an α-helical hairpin (blue) comprised of α4 and α5. Hairpin terminal residues Arg53, Gln66, Ile125, and Glu149 are shown as spacefilling atoms and are equivalent to residues 34, 47, 106, and 130, respectively, at the homologous hairpin termini in Kv1.2. (b). Amino acid sequence of the T1 Kv1.3 β–hairpin (red) and α-helical hairpin (blue) with secondary structure assignments derived from the highly identical Kv1.2 structure in (a)14. Engineered cysteines 53C and 66C (β–hairpin) and 125C and 149C (α-helical hairpin) in Kv1.3 are highlighted in yellow. The PTC is indicated by the vertical black bar at the right.